THE GROWTH AND PHYSIOLOGICAL RESPONSES OF TWO GREEN ALGAE TO THE CHANGE OF CO2 CONCENTRATION
-
摘要: 以小球藻FACHB-1580和栅藻FACHB-1618为研究对象, 比较了两株绿藻在0.04% CO2、5% CO2和20% CO2 (v/v)三种通气培养条件下的生长和生理特性的响应, 试图阐述与无机碳利用相关生理参数和微藻利用CO2能力的关系。结果表明, 两株绿藻均能高效利用CO2, 在5%(v/v)条件下均表现出最大生物量积累、最大比生长速率和最大二氧化碳固定速率。小球藻FACHB-1580和栅藻FACHB-1618最大生物量分别为3.5和5.4 g/L, 分别是0.04% CO2 (v/v)条件的1.41和1.46倍。在高达20% CO2 (v/v)条件下, 两株绿藻的生物量均显著高于空气组(P<0.05)。随着CO2浓度的增加, 两株绿藻的无机碳亲和力、胞内和胞外CA活性、初始Rubisco活性, 及Rubisco活化度均有下降趋势, 总的Rubisco活性变化不明显。另外, 小球藻FACHB-1580存在较高的胞外和胞内CA活性; 而栅藻FACHB-1618胞外CA活性几乎为零, 胞内CA活性显著低于小球藻FACHB-1580。由此推测, 小球藻FACHB-1580能同时吸收介质中的
${\rm{HCO}}_3^ - $ 和CO2, 其胞内CA催化胞内${\rm{HCO}}_3^ - $ 快速转化为CO2, 从而为Rubisco提供充足的CO2来源; 而栅藻FACHB-1618主要吸收介质中的CO2, 其胞内CA活性较低, 推测其通过提高胞内CA含量, 或增强Rubisco对CO2的亲和力等促进光合固碳作用。-
关键词:
- 绿藻 /
- 生物量 /
- 二氧化碳浓缩机制(CCM) /
- 碳酸酐酶(CA) /
- 1,5-二磷酸核酮糖羧化酶(Rubisco)
Abstract: Selection of microalgae strains that are capable of utilizing high concentration of CO2 is essential for industrial application. At present, the microalgae research mainly focus on the biomass, bioactive products and recovery properties, rather than physiological properties. The influence of different CO2 concentrations, including air, 5% CO2 and 20% CO2 (v/v), on the growth and physiological properties of Chlorella sp. FACHB-1580 and Scenedesmus sp. FACHB-1618 were studied. The goal was to clarify the relationship between the physiological properties and the CO2 utilizing capacity. Results showed that both strains were capable to use high concentration of CO2 and had the maximum biomass accumulation, specific growth rate and CO2 fixation rate under 5% CO2 (v/v) culture condition. The maximum biomass was 3.5 g/L in Chlorella sp. FACHB-1580 and 5.4 g/L in Scenedesmus sp. FACHB-1618 which was 1.41 and 1.46 times higher in comparison with the control (air) group, respectively. Both strains had higher biomass under 20% CO2 (v/v) condition than that in the air group (P < 0.05). With the increasing supply of CO2, the affinity of inorganic carbon, the activity of intracellular (CAint) and extracellular (CAext) carbonic anhydrase, as well as the initial activity and activation rate of Rubisco declined in both strains. However, there was no significant difference in the total Rubisco activity across time. In addition, Chlorella sp. FACHB-1580 had significantly higher levels of CAint and CAext activities. By contrast, Scenedesmus sp. FACHB-1618 had very poor CAint and CAext activities and were not detectable, indicating these two strains differed significantly in the utility of inorganic carbon. It was speculated that Chlorella sp. FACHB-1580 can absorb both CO2 and${\rm{HCO}}_3^ - $ whereas the Scenedesmus sp. FACHB-1618 tends to only absorb CO2. Chlorella sp. FACHB-1580 may obtain its abundant CO2 for Rubisco via high CAint activity; for Scenedesmus sp. FACHB-1618, due to its lower CAint activity, it may increase the amount of CA protein, or higher carboxylase affinity for CO2 to promote the reactions of photosynthesis. -
微藻是一类单细胞或简单多细胞光合自养型生物, 其数量多、分布广、生长快、繁殖周期短, 因而能高效固定CO2并释放氧气, 平均1 kg藻细胞干重可固定1.8 kg CO2。微藻不仅是高效固定CO2的“工厂”, 藻体还可作为生物能源、食物、饲料、生物活性提取物等原料为人类所用[1]。随着能源危机和环境恶化, 利用微藻减排已成为国际CO2减排和新能源开发领域的前沿研究热点和高技术竞争点[2]。工业排放废气中CO2浓度往往高达10%—15%(v/v)[3], 因此, 筛选能够有效利用高浓度CO2的优良藻种是实现微藻减排的关键。在微藻培养过程中, CO2供给浓度会影响培养液中无机碳的组成, 从而影响藻株对无机碳利用的机制和生长情况。研究表明, 不同藻种对CO2的适应性不同, 如: 小球藻Chlorella sp.和微拟球藻Nannochloropsis oculata在高于5% CO2 (v/v)条件下生长受到抑制[4]。相反地, 莱茵衣藻Chlamydomonas reinhardtii在5% CO2 (v/v)条件下的最大比生长速率是空气组的2倍[5]。
This page contains the following errors:
error on line 1 at column 1: Start tag expected, '<' not foundBelow is a rendering of the page up to the first error.
藻细胞吸收无机碳后, 通过Calvin-Benson循环(卡尔文循环)固定CO2, 最终转化为有机物储存。1,5-二磷酸核酮糖羧化酶(D-ribulose 1,5-biphosphate carboxylase, 简写为Rubisco, EC 4.1.1.39)是卡尔文循环的关键酶, 它催化1分子二磷酸核酮糖(RuBp)与1分子CO2结合产生2分子3-磷酸甘油酸(PGA)。该反应是光合固碳的限速步骤, 决定着光合作用产物的积累。与一般的酶相比, Rubisco具有2个显著特征: 一是非专一性, 即Rubisco能同时催化羧化反应和加氧反应; 二是低效性[11], 这也是藻株诱导CCM的另一个重要原因。CO2不仅是Rubisco催化的底物, 还是Rubisco的活化剂和活性调节因子[12]。大量报道表明, CO2浓度会影响Rubisco的特性及其蛋白的合成[13—15], 了解CO2供给浓度对微藻Rubisco的影响, 有助于更好把握微藻的生长特性。
本研究以筛选用于耦合烟道气CO2减排的户外规模培养的潜在优良藻种为出发点, 比较小球藻FACHB-1580和栅藻FACHB-1618在不同CO2供给条件下的生长和生理响应。试图阐述与无机碳利用相关生理参数和微藻利用CO2能力的关系, 并探索有效表征微藻固定CO2能力的生理指标, 从而为高效固碳优良藻种的筛选和评价提供指导依据。
1. 材料与方法
1.1 藻种来源及培养条件
实验用到的栅藻(Scendesmus sp. FACHB-1618)和小球藻(Chlorella sp. FACHB-1580)均来自中国科学院淡水藻种库(FACHB-Collection, Wuhan, China)。
培养装置为内径5 cm, 有效体积750 mL的柱状光生物反应器, 培养条件为: BG11无碳培养基[16](去除Na2CO3, 以同等摩尔Na+浓度的NaCl代替), 初始光照强度约为50 μmol/(m2·s), 依据藻细胞密度进行调节, 持续光照, 温度(25±2)℃。
通气条件为0.04%、5%和20% CO2 (v/v)三组, 每组3个平行, 气体流量为200 mL/min, 连续通气。不同浓度CO2由空气和纯CO2混合而成, 用CO2测定仪(MS400, Eranntex, Shenzhen, China)测定CO2浓度。藻液初始接种浓度为A680=0.3±0.1, 培养周期17d。
1.2 生物量测定
生物量以藻细胞干重(Dry Weight, DW)表示, 接种后每2—3d取5 mL藻液, 用玻璃纤维滤膜(1.2 μm, Whatman GF/C, GE Healthcare, Chicago, IL, USA)过滤。过滤前后, 滤膜在105℃, 2h烘干至恒重, 称重并记录重量依次为W0 (g), Wt (g)。相关计算公式如下:
生物量: X (g/L)=(W0–Wt)/5×1000
生物量产率: P [mg/(L·d)]=(X2–X1)/(t2–t1)×1000
比生长速率: μ=(lgX2–lgX1)/(t2–t1)×3.332
This page contains the following errors:
error on line 1 at column 1: Start tag expected, '<' not foundBelow is a rendering of the page up to the first error.
This page contains the following errors:
error on line 1 at column 1: Start tag expected, '<' not foundBelow is a rendering of the page up to the first error.
1.3 表观无机碳亲和力常数K0.5(DIC)的测定
收集对数生长期藻细胞, 去上清, 重悬于预备的无碳缓冲液BG11-BTP(pH 8.0)[17]中, 用该缓冲液清洗细胞3次。清洗后的细胞液取出一部分测定叶绿素; 另外取出2 mL置于液相氧电极(Chlorlab2, Hansatech Instruments Ltd., Norfolk, UK)反应槽内, 在饱和光强500 μmol/(m2·s)下, 利用藻细胞光合固碳作用使胞内无机碳枯竭, 当藻细胞光合放氧速率几乎为零时, 通过微量进样器从加样孔加入不同量的NaHCO3溶液, 测定藻细胞光合放氧速率。藻株对无机碳的亲和力用无机碳半饱和常数K0.5 (DIC) (即光合作用速率达到一半时的无机碳浓度)来表征, 并通过方程V=Vmax[DIC]/(K0.5+[DIC])对实验数据进行非线性拟合后求得[18]。
1.4 Rubisco活性测定
收集对数期藻液(干重约20 mg), 5000 r/min, 25℃离心5min, 去上清后用pH 7.5 Tris-HCl清洗两遍。在4℃条件下, 加900 μL提取液(50 mmol/L Tris-HCl pH 7.5, 1 mmol/L EDTA pH 8.0, 10 mmol/L MgCl2, 12% (v/v) Glycerol, 1% (w/v) PVP 40, 2.5 mmol/L DTT), 将细胞转移到2 mL冻存管中, 并加入0.5 g玻璃珠/锆珠(直径0.5 mm); 利用低温细胞破碎仪(Gene Ready, Bioeer, Beijing, China)破碎细胞后, 12000 r/min, 4℃离心10min, 上清即Rubisco粗提液。粗提液一部分直接用于初始Rubisco活性的测定, 一部分需用活化液(33 mmol/L Tris-HCl pH 7.5, 0.67 mmol/L EDTA, 33 mmol/L MgCl2, 10 mmol/L NaHCO3)活化后测定总的Rubisco活性[19]。Rubisco的活化度为初始Rubisco活性与总的Rubisco活性的比值。
利用分光光度计法测定Rubisco活性[20], 并根据催化反应过程中所消耗的NADH的量来计算该酶活性[21], 测定温度均为30℃。具体方法是: 取5 μL粗提液/活化酶液加入187 μL反应液(50 mmol/L HEPES-KOH pH 8.0, 1 mmol/L EDTA pH 8.0, 20 mmol/L MgCl2, 2.5 mmol/L DTT, 10 mmol/L NaHCO3, 5 mmol/L ATP, 5 mmol/L Phosphocreatine, 0.2 mmol/L NADH, 10 U/mL PGK, 10 U/mL GADPH, 10 U/mL PCK)中, 最后加入8 μL 50 mmol/L RuBp, 使其终浓度达到2 mmol/L。立即测定A340的变化, 每30s测一次, 连续10min, 计算A340的变化率。采用氨基黑方法[22]测定粗提液中蛋白质含量。
酶比活力: U=10×ΔA340/6.22/2 (mg pro)
1.5 CA活性测定
收集对数期的藻细胞(干重约20 mg), 用20 mmol/L巴比妥钠(CAS:144-02-5)缓冲液(pH 8.3)清洗藻细胞两遍并重悬; 重悬液直接用于胞外CA活性测定, 总的CA活性用低温细胞破碎仪(Gene Ready, Bioeer, Beijing, China)破碎细胞后测定, 胞内CA活性即总的CA与胞外CA活性的差值。
采用量电法[23]测定CA活性。取缓冲液清洗过的细胞液测定叶绿素含量, 利用甲醇加热法[24]提取叶绿素a。计算方法如下:
叶绿素a含量: Chl.a (mg/L)=15.56×A666–7.34×A653[25]
CA酶活性: EU=10×(T0/T–1)/(μg Chl.a)
T0: 不含CA的巴比妥钠缓冲液pH从8.30降到7.30的时间(对照组);
T: 含有CA的巴比妥钠缓冲液pH从8.30降到7.30的时间(实验组)。
1.6 数据分析
实验数据采用平均值±标准差表示, 利用SPSS 18.0统计软件分析各组数据的统计学差异, 显著水平为P<0.05, 极显著水平为P<0.01。
2. 结果
2.1 两株绿藻在不同浓度CO2通气培养条件下生长的比较
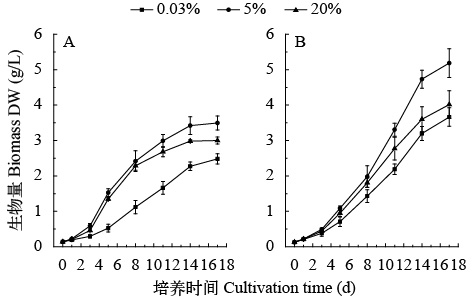
两株绿藻均在5% CO2 (v/v)条件下获得最大生物量积累、最大比生长速率和最大CO2固定速率。经通气培养17d后, 小球藻FACHB-1580和栅藻FACHB-1618最大生物量达到3.5和5.4 g/L(图 1), 分别是空气组的1.41和1.46倍。两株绿藻最大固碳速率和最大比生长速率分别是563.7和660.9 mg/(L·d), 0.78和0.57/d(表 1)。同时, 两株绿藻均能适应20% CO2 (v/v)条件, 该条件下的比生长速率与0.04% CO2 (v/v)组相比分别增加83%和22%, 且该条件下小球藻FACHB-1580的生物量显著高于0.04% CO2 (v/v)组(P<0.05)。
表 1 两株绿藻在不同浓度CO2通气培养条件下的生长参数(平均值±标准差)Table 1. Growth parameters of two strains under different CO2 concentrations (Mean±SD)CO2浓度CO2 conc.(v/v) FACHB-1580 FACHB-1618 比生长速率 μmax(/d) CO2固定速率 $ {R_{{\rm{C}}{{\rm{O}}_2}}} $ [mg/(L·d)]
比生长速率 μmax(/d) CO2固定速率 $ {R_{{\rm{C}}{{\rm{O}}_2}}} $ [mg/(L·d)]
0.04% CO2 0.48±0.04 321.3±46.7 0.45±0.05 425.1±15.9 5% CO2 0.78±0.06** 563.7±40.3** 0.57±0.02* 660.9±33.7** 20% CO2 0.77±0.009** 521.9±33.7** 0.55±0.04* 546.9±73.9* 注: *P<0.05, **P<0.01, 下同Note: *P<0.05, **P<0.01, the same applies below 2.2 两株绿藻在不同浓度CO2通气培养条件下无机碳亲和力的比较
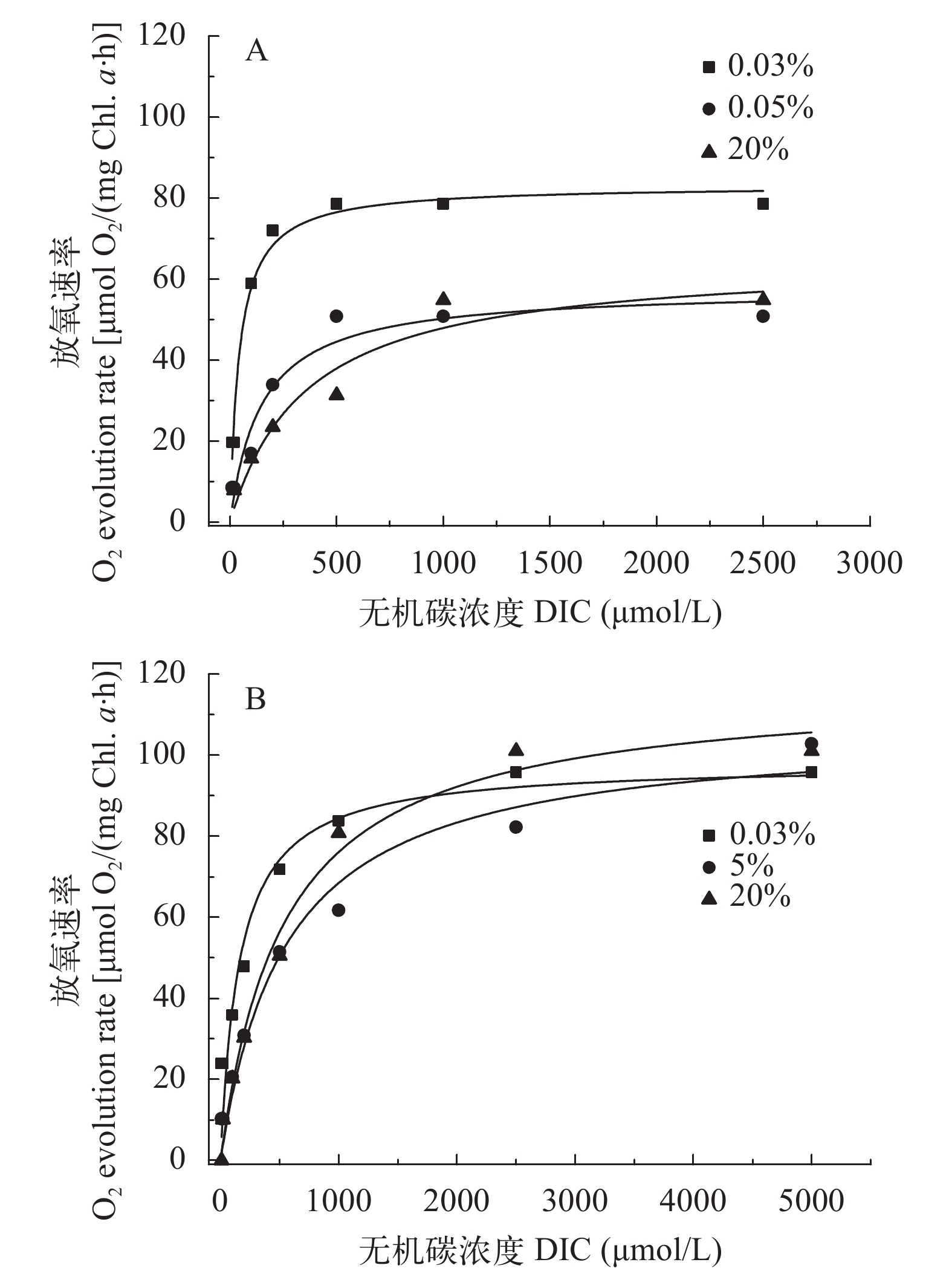
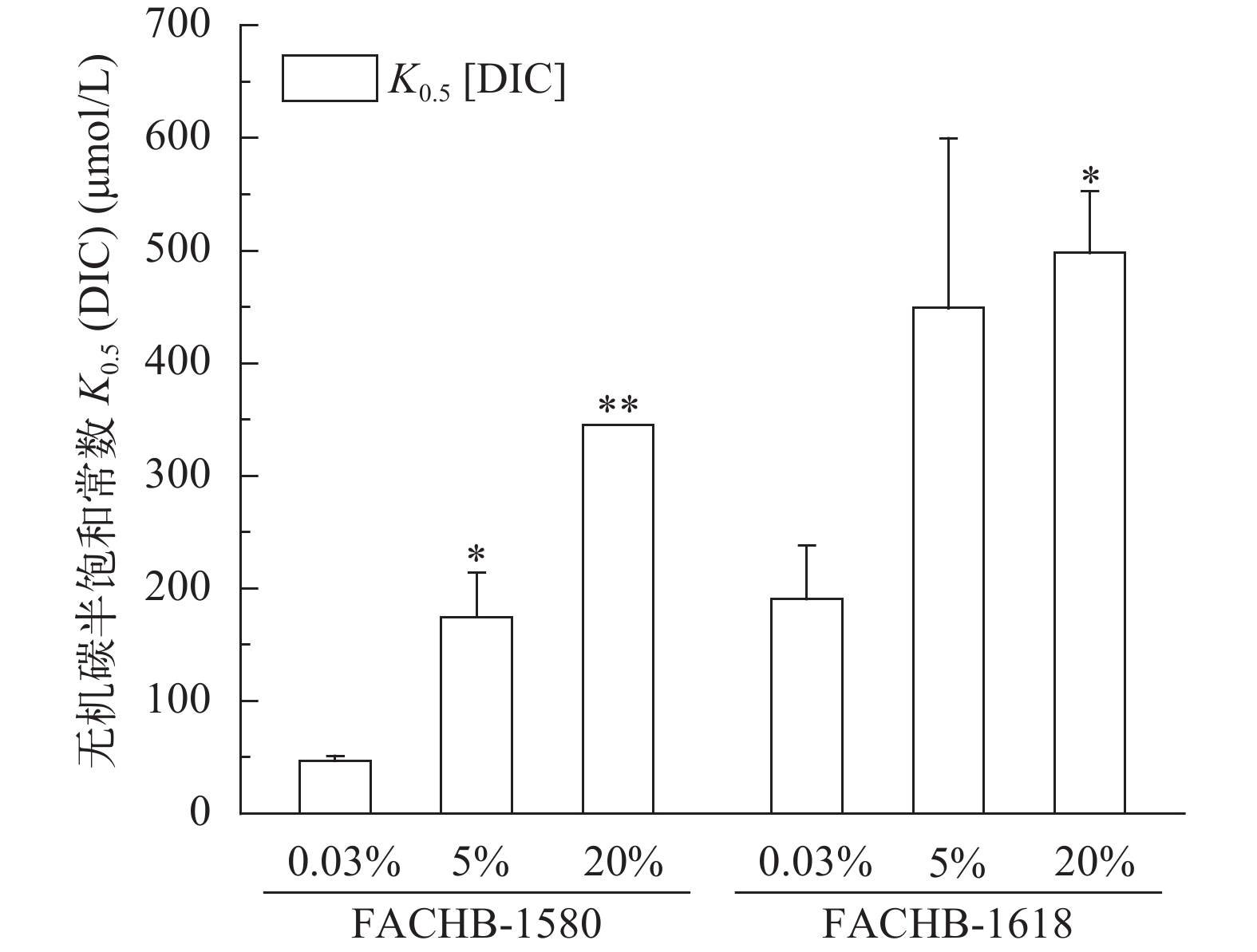
图 2表示两株绿藻在饱和光强500 μmol/(m2·s)条件下的无机碳吸收曲线, 吸收曲线反应它们在不同浓度CO2培养条件下最大光合放氧速率变化不大。但它们的表观无机碳亲和力常数K0.5 (DIC)均随着CO2浓度增加而增高(图 3)。小球藻FACHB-1580的K0.5 (DIC)分别为50.6、174.6和344.8 μmol/L, 20% CO2 (v/v)组的K0.5 (DIC)是0.04% CO2 (v/v)组的6.8倍; 栅藻FACHB-1618的K0.5 (DIC)分别为190.6、443.9和498.1 μmol/L, 20% CO2 (v/v)组的K0.5 (DIC)是0.04% CO2 (v/v)组的2.6倍(表 2)。另外, 在同等条件下, 小球藻FACHB-1580的K0.5(DIC)值均低于栅藻FACHB-1618, 即在同等条件下小球藻FACHB-1580的无机碳亲和力高于栅FACHB-1618。
表 2 两株绿藻在不同浓度CO2培养条件下无机碳亲和力常数K0.5 (DIC)、最大光合放氧速率Vmax的比较(平均值±标准差)Table 2. K0.5(DIC) and the maximum photosynthesis rate of two strains under different CO2 concentrations (Mean±SD)通气浓度CO2 conc.(v/v) 最大光合放氧速率 Vmax [μmol O2/(mg Chl.a·h)] 无机碳亲和力常数 K0.5(DIC)(μmol/L) FACHB-1580 FACHB-1618 FACHB-1580 FACHB-1618 0.04% CO2 81.1±10.0 102.1±9.4 50.6±0.8 190.6±47.3 5% CO2 58.7±1.4* 103.7±3.5 174.6±39.3* 443.9±143.6 20% CO2 62.9±2.3 112.8±6.0 344.8±0** 498.1±54.8* 2.3 两株绿藻在不同浓度CO2通气培养条件下CA活性的比较
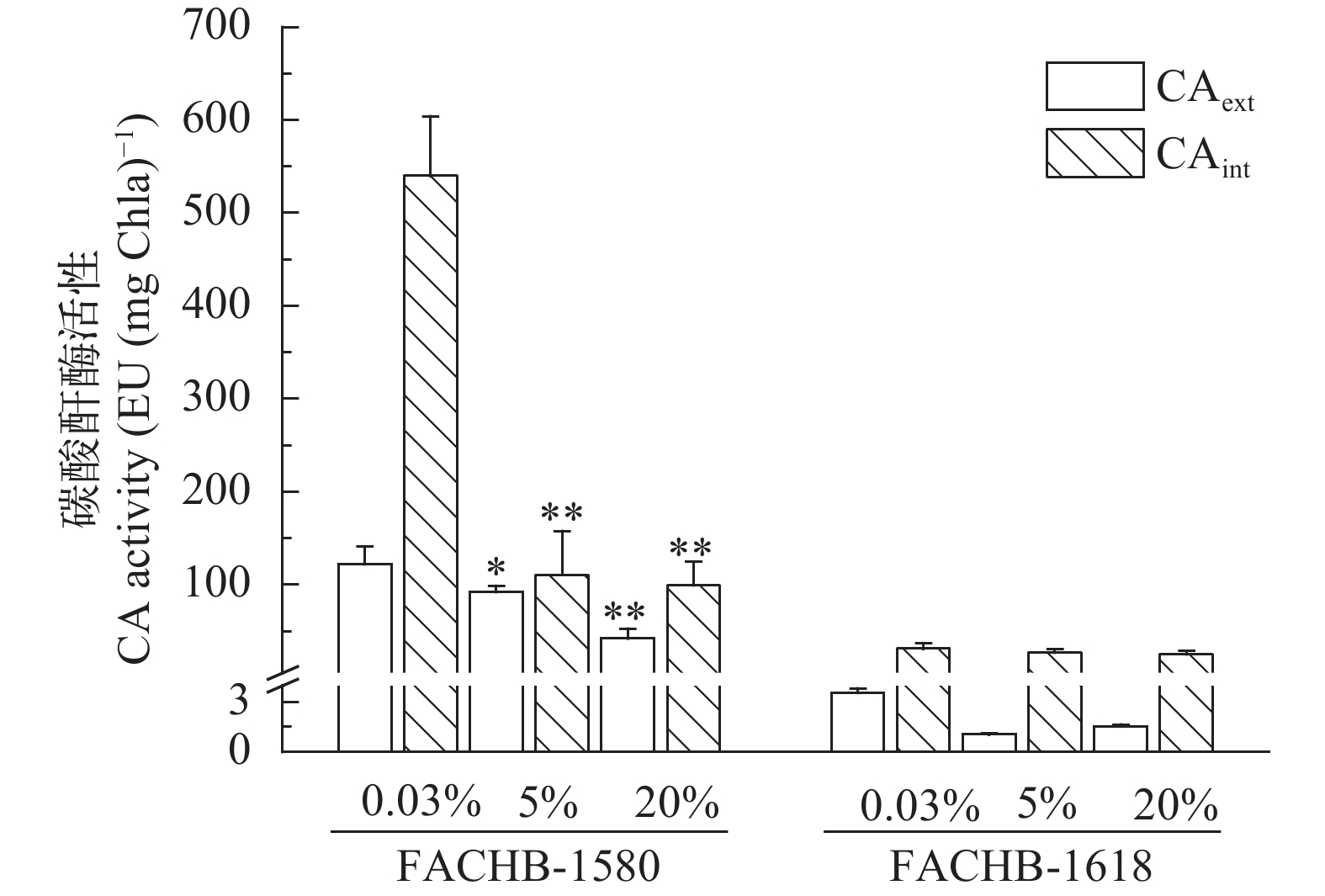
小球藻FACHB-1580存在胞外CA, 其活性随CO2浓度增加显著降低; 而栅藻FACHB-1618胞外CA活性极低(≤ 3.6 EU/mg Chl.a), 5%和20% CO2 (v/v)条件下几乎为零(图 4)。另外, 小球藻FACHB-1580的胞内CA活性较高, 且随CO2浓度增加其活性显著降低(P<0.01)。其中在0.04% CO2 (v/v)条件下胞内CA活性为540.0 EU/(mg Chl.a), 是20% CO2 (v/v)组的5.4倍。小球藻FACHB-1580高活性的胞内CA为Rubisco提供了充足的CO2来源。相比而言, 栅藻FACHB-1618胞内CA活性较低, 随着CO2浓度的变化, 其活性变化不明显。
2.4 两株绿藻在不同浓度CO2通气培养条件下Rubisco活性的比较
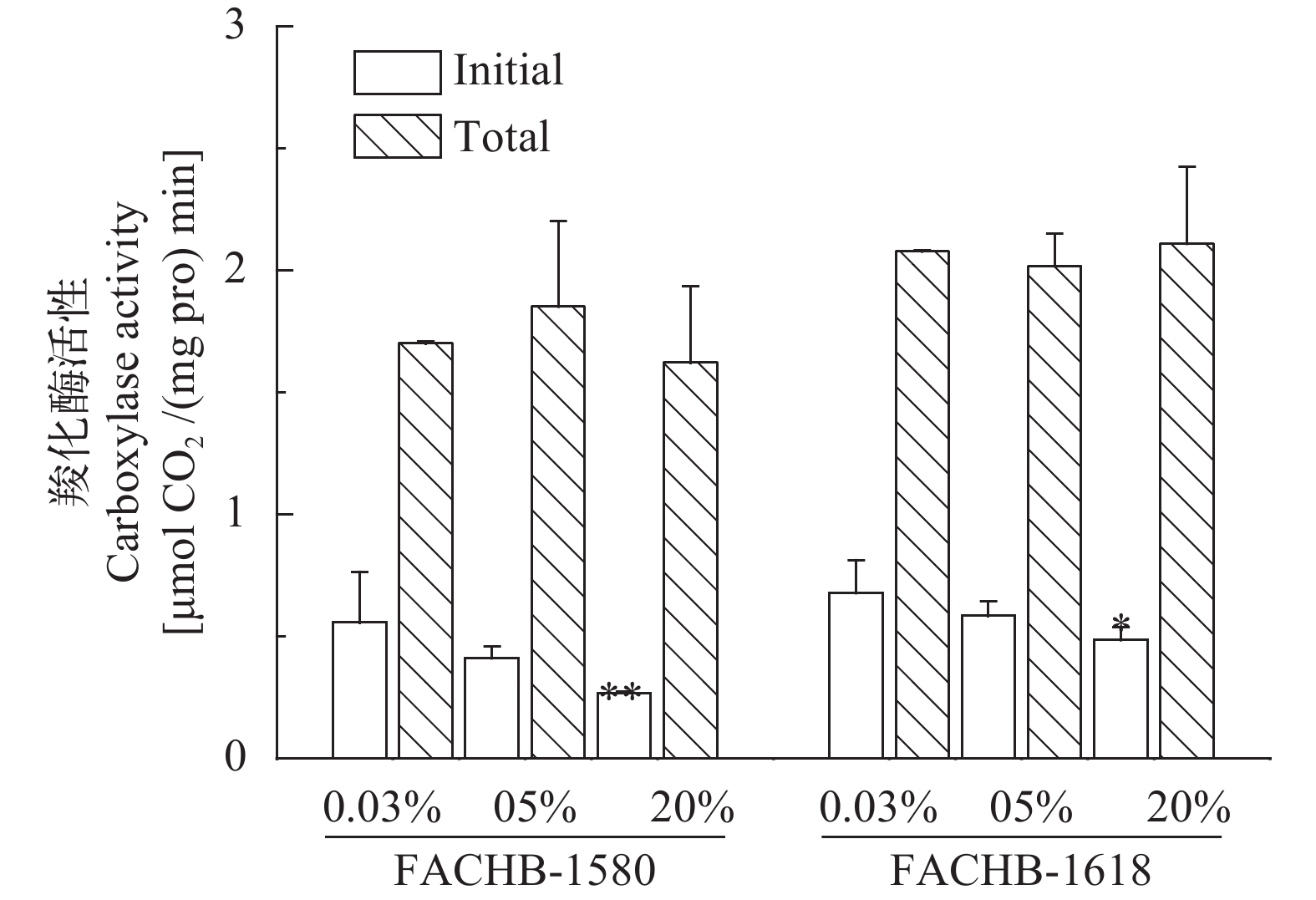
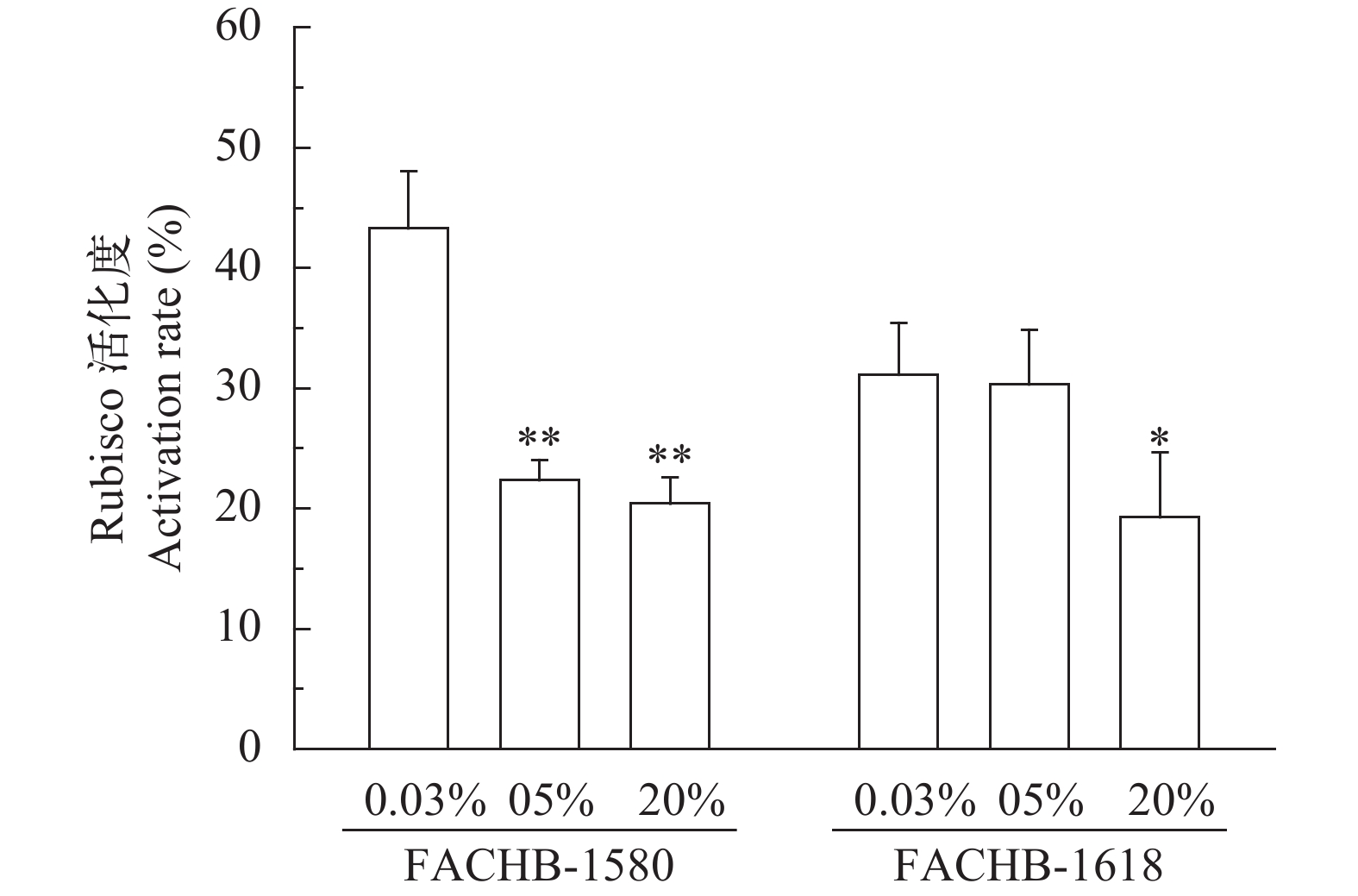
随着CO2浓度的增加, 两株绿藻初始Rubisco活性和Rubisco活化度均有下降趋势(图 5、图 6), 而总的Rubisco活性无明显变化。在0.04%、5%和20% CO2 (v/v)下, 小球藻FACHB-1580的初始Rubisco活性分别为0.56、0.4和0.27 μmol CO2/(mg pro·min), 对应Rubisco活化度分别为43%、22%和20%; 栅藻FACHB-1618的初始Rubisco活性分别为0.68、0.58和0.49 μmol CO2/(mg pro·min), 对应Rubisco活化度分别为31%、30%和19%。20% CO2 (v/v)条件下, 两株绿藻初始Rubisco活性及Rubisco活化度与0.04% CO2 (v/v)组相比均显著降低(P<0.05)。
3. 讨论
大量研究表明, 和空气条件相比, CO2供给浓度的增加, 往往能够促进藻细胞快速生长[26, 27]。然而, 持续高浓度CO2的通入会迅速降低溶液pH, 可能抑制相关酶活性[27]。大多数藻株适宜生长的CO2浓度范围是0.038%—10%(v/v)[28]。小球藻(Chlorella sp.)只能在低于2% CO2 (v/v)条件下生长[4]。少数藻种, 如: 微拟球藻(Nannochloropsis sp.)在15% CO2 (v/v)条件下比生长速率为0.52/d, 相比空气条件提高58%[26]。在本文研究中, 小球藻FACHB-1580和栅藻FACHB-1618均在5% CO2 (v/v)条件下有最大生物量积累、最大比生长速率, 及最大CO2固定速率。在20% CO2 (v/v)条件下, 它们的比生长速率与0.04% CO2 (v/v)组相比分别增加83%和22%, 表明实验所用两株绿藻均能适应体积比高达20%的CO2供给条件并高效固定CO2用于生物量积累。尽管栅藻FACHB-1618的生物量高于小球藻FACHB-1580, 但从比生长速率来看, 小球藻FACHB-1580对20% CO2 (v/v)条件的适应性更强。
不同浓度CO2通气培养条件下藻株的生长与其对培养液中无机碳的利用机制紧密相关。已有研究表明, 微藻通常在低碳条件下开启CCM, 高碳条件下CCM受抑制[29], 表现为表观无机碳亲和力常数增加, 即无机碳亲和力显著下降[30, 31]。聚球藻Synechococcus sp.在2% CO2 (v/v)条件下K0.5(DIC)与0.35% CO2条件相比升高了20倍[32]; 集胞藻Synechocystis sp.在2% CO2 (v/v)条件下的K0.5(DIC)是0.02% CO2条件的5.4倍[33]。在本实验中, 两株绿藻的K0.5(DIC)值均随CO2供给浓度的增加而升高(图 4), 该结果与前人研究结果一致。
This page contains the following errors:
error on line 1 at column 1: Start tag expected, '<' not foundBelow is a rendering of the page up to the first error.
This page contains the following errors:
error on line 1 at column 1: Start tag expected, '<' not foundBelow is a rendering of the page up to the first error.
综上, 本研究较全面地探讨了两株绿藻在不同浓度CO2通气培养条件下的生长和生理特性的响应, 对今后藻种的筛选和评价具有一定指导意义。同时, 本研究的两株藻种均能够高效利用CO2, 有望成为耦合烟道气减排的户外规模培养的潜在优良藻株。但是, 本研究结果还不足以形成有效地指征藻株对CO2利用能力的评价体系。为此, 我们需要结合烟道气的特点, 进一步探讨藻株对烟道气中有毒气体(SOx/NOx)、粉尘等因素的耐受能力。并将实验室所选藻株在室外进行扩大培养, 得到更多藻株户外大规模培养的生长、生理参数等。
-
表 1 两株绿藻在不同浓度CO2通气培养条件下的生长参数(平均值±标准差)
Table 1 Growth parameters of two strains under different CO2 concentrations (Mean±SD)
CO2浓度CO2 conc.(v/v) FACHB-1580 FACHB-1618 比生长速率 μmax(/d) CO2固定速率 $ {R_{{\rm{C}}{{\rm{O}}_2}}} $ [mg/(L·d)]
比生长速率 μmax(/d) CO2固定速率 $ {R_{{\rm{C}}{{\rm{O}}_2}}} $ [mg/(L·d)]
0.04% CO2 0.48±0.04 321.3±46.7 0.45±0.05 425.1±15.9 5% CO2 0.78±0.06** 563.7±40.3** 0.57±0.02* 660.9±33.7** 20% CO2 0.77±0.009** 521.9±33.7** 0.55±0.04* 546.9±73.9* 注: *P<0.05, **P<0.01, 下同Note: *P<0.05, **P<0.01, the same applies below 表 2 两株绿藻在不同浓度CO2培养条件下无机碳亲和力常数K0.5 (DIC)、最大光合放氧速率Vmax的比较(平均值±标准差)
Table 2 K0.5(DIC) and the maximum photosynthesis rate of two strains under different CO2 concentrations (Mean±SD)
通气浓度CO2 conc.(v/v) 最大光合放氧速率 Vmax [μmol O2/(mg Chl.a·h)] 无机碳亲和力常数 K0.5(DIC)(μmol/L) FACHB-1580 FACHB-1618 FACHB-1580 FACHB-1618 0.04% CO2 81.1±10.0 102.1±9.4 50.6±0.8 190.6±47.3 5% CO2 58.7±1.4* 103.7±3.5 174.6±39.3* 443.9±143.6 20% CO2 62.9±2.3 112.8±6.0 344.8±0** 498.1±54.8* -
[1] Chisti Y. Biodiesel from microalgae [J]. Biotechnology Advances, 2007, 25(3): 294—306
[2] Rahaman M S A, Cheng L H, Xu X H, et al. A review of carbon dioxide capture and utilization by membrane integrated microalgal cultivation processes [J]. Renewable and Sustainable Energy Reviews, 2011, 15(8): 4002—4012
[3] De Morais M G, Costa J A V. Biofixation of carbon dioxide by SpirμLina sp. and Scenedesmus obliquus cultivated in a threestage serial tubμLar photobioreactor [J]. Journal of Biotechnology, 2007, 129(3): 439—445
[4] Chiu S Y, Kao C Y, Chen C H, et al. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor [J]. Bioresource Technology, 2008, 99(9): 3389—3396
[5] Förster B, Osmond C B, Boynton J E. Very high light resistant mutants of Chlamydomonas reinhardtii: responses of photosystem Ⅱ, nonphotochemical quenching and xanthophyll pigments to light and CO2 [J]. Photosynthesis Research, 2001, 67(1—2): 5—15
[6] Moroney J V, Ynalvez R A. Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii [J]. Eukaryotic Cell, 2007, 6(8): 1251—1259
[7] Gerloff E A, Spijkerman E, Pröschold T. Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acidic lake (pH 2.6) [J]. Plant, Cell & Environment, 2005, 28(10): 1218—1229
[8] Spijkerman E, Stojkovic S, Beardall J. CO2 acquisition in Chlamydomonas acidophila is influenced mainly by CO2, not phosphorus, availability [J]. Photosynthesis Research, 2014, 121(2—3): 213—221
[9] Tsuzuki M, Miyachi S. CO2 syndrome in Chlorella [J]. Canadian Journal of Botany, 1991, 69(5): 1003—1007
[10] Moroney J V, Kitayama M, Togasaki R K, et al. Evidence for inorganic carbon transport by intact chloroplasts of Chlamydomonas reinhardtii [J]. Plant Physiology, 1987, 83(3): 460—463
[11] Ashida H, Danchin A, Yokota A. Was photosynthetic Rubisco recruited by acquisitive evolution from Rubisco-like proteins involved in sulfur metabolism [J]? Research in Microbiology, 2005, 156(5—6): 611—618
[12] Bowes G. Growth at elevated CO2: photosynthetic responses mediated through Rubisco [J]. Plant, Cell & Environment, 1991, 14(8): 795—806
[13] Spijkerman E. What physiological acclimation supports increased growth at high CO2 conditions [J]. Physiologia Plantarum, 2008, 133(1): 41—48
[14] Price G D, Coleman J R, Badger M R. Association of carbonic anhydrase activity with carboxysomes isolated from the cyanobacterium Synechococcus PCC7942 [J]. Plant Physiology, 1992, 100(2): 784—793
[15] Rogers A, Ellsworth D S. Photosynthetic acclimation of Pinus taeda (loblolly pine) to long-term growth in elevated pCO2 (FACE) [J]. Plant, Cell & Environment, 2002, 25(7): 851—858
[16] Rippka R, Deruelles J, Waterbury J B, et al. Generic assignments, strain histories and properties of pure cultures of cyanobacteria [J]. Microbiology, 1979, 111(1): 1—61
[17] Qiu B S, Gao K S. Effects of CO2 enrichment on the bloom-forming cyanobacterium Microcystis aeruginosa (cyanophyceae): Physiological responses and relationships with the availability of dissolved inorganic carbon1 [J]. Journal of Phycology, 2002, 38(4): 721—729
[18] Rotatore C, Colman B. The acquisition and accumulation of inorganic carbon by the unicellular green alga Chlorella ellipsoidea [J]. Plant, Cell & Environment, 1991, 14(4): 377—382
[19] Mouget J L, Beeson Jr R C, Legendre L, et al. Inadequacy of Rubisco initial and total activities to account for observed rates of photosynthetic carbon dioxide assimilation by Scenedesmus ecornis [J]. European Journal of Phycology, 1993, 28(2): 99—106
[20] Gerard V A, Driscoll T. A spectrophotometric assay for Rubisco activity: application to the kelp laminaria saccharrina and implications for radiometric assays [J]. Journal of Phycology, 1996, 32(5): 880—884
[21] Eckardt N A, Snyder G W, Portis Jr A R, et al. Growth and photosynthesis under high and low irradiance of Arabidopsis thaliana antisense mutants with reduced ribulose-1, 5-bisphosphate carboxylase/oxygenase activase content [J]. Plant Physiology, 1997, 113(2): 575—586
[22] Kaplan R S, Pedersen P L. Determination of microgram quantities of protein in the presence of milligram levels of lipid with amido black 10B1 [J]. Analytical Biochemistry, 1985, 150(1): 97—104
[23] Wilbur K M, Anderson N G. Electrometric and colorimetric determination of carbonic anhydrase [J]. Journal of Biological Chemistry, 1948, 176(1): 147—154
[24] Pruvost J, van Vooren G, Le Gouic B, et al. Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application [J]. Bioresource Technology, 2011, 102(1): 150—158
[25] Lichtenthaler H, Wellburn A R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents [J]. Biochemical Society Transactions, 1983, 11: 591—592
[26] Jiang L, Luo S, Fan X, et al. Biomass and lipid production of marine microalgae using municipal wastewater and high concentration of CO2 [J]. Applied Energy, 2011, 88(10): 3336—3341
[27] Tang D, Han W, Li P, et al. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels [J]. Bioresource Technology, 2011, 102(3): 3071—307
[28] Chiu S Y, Kao C Y, Huang T T, et al. Microalgal biomass production and on-site bioremediation of carbon dioxide, nitrogen oxide and sulfur dioxide from flue gas using Chlorella sp. Cultures [J]. Bioresource Technology, 2011, 102(19): 9135—9142
[29] Giordano M, Beardall J, Raven J A. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution [J]. Annual Review of Plant Biology, 2005, 56: 99—131
[30] Yu J W, Price G D, Badger M R. A mutant isolated from the cyanobacterium Synechococcus PCC7942 is unable to adapt to low inorganic carbon conditions [J]. Plant Physiology, 1994, 104(2): 605—611
[31] Sültemeyer D, Price G D, Yu J W, et al. Characterisation of carbon dioxide and bicarbonate transport during steady-state photosynthesis in the marine cyanobacterium Synechococcus strain PCC7002 [J]. Planta, 1995, 197(4): 597—607
[32] Price G D, Maeda S, Omata T, et al. Modes of active inorganic carbon uptake in the cyanobacterium, Synechococcus sp. PCC7942 [J]. Functional Plant Biology, 2002, 29(3): 131—149
[33] Benschop J J, Badger M R, Dean Price G. Characterisation of CO2 and
${\rm{HCO}}_3^ - $ uptake in the cyanobacterium Synechocystis sp. PCC6803 [J]. Photosynthesis Research, 2003, 77(2—3): 117—126[34] 韩博平, 韩志国, 付翔. 藻类光合作用机理与模型, 北京: 科学出版社. 2003 Han B P, Han Z G, Fu X. Algal photosynthesis: mechanisms and models [M]. Beijing: Science Press. 2003
[35] Miyachi S, Iwasaki I, Shiraiwa Y. Historical perspective on microalgal and cyanobacterial acclimation to low- and extremely high-CO2 conditions [J]. Photosynthesis Research, 2003, 77(2—3): 139—153
[36] Swarnalatha G V, Hegde N S, Chauhan V S, et al. The effect of carbon dioxide rich environment on carbonic anhydrase activity, growth and metabolite production in indigenous freshwater microalgae [J]. Algal Research, 2015, 9: 151—159
[37] Burkhardt S, Amoroso G, Riebesell U, et al. CO2 and
${\rm{HCO}}_3^ - $ uptake in marine diatoms acclimated to different CO2 concentrations [J]. Limnology and Oceanography, 2001, 46(6): 1378—1391[38] Chen X, Gao K. Effect of CO2 concentrations on the activity of photosynthetic CO2 fixation and extracelluar carbonic anhydrase in the marine diatom Skeletonema costatum [J]. Chinese Science Bulletin, 2003, 48(23): 2616—2620
[39] Wu H, Gao K. Ultraviolet radiation stimulated activity of extracellular carbonic anhydrase in the marine diatom Skeletonema costatum [J]. Functional Plant Biology, 2009, 36(2): 137—143
[40] 高坤山. 藻类固碳理论、进展与方法. 见: 邹定辉, 褐藻. 北京: 科学出版社. 2014, 200—205 Gao K S. Algae Carbon FixationBase, Advance and Methods [A]. In: Zou D H (Eds.), Brown Algae [C]. Beijing: Science Press. 2014, 200—205
[41] Griffin K L, Tissue D T, Turnbull M H, et al. The onset of photosynthetic acclimation to elevated CO2 partial pressure in field-grown Pinus radiata D. Don. after 4 years [J]. Plant, Cell & Environment, 2000, 23: 1089—1098
[42] Rogers A, Ellsworth D S. Photosynthetic acclimation of Pinus taeda (Loblolly pine) to longterm growth in elevated pCO2 (FACE) [J]. Plant, Cell & Environment, 2002, 25: 851—858
[43] Sage R F, Sharkey T D, Seemann J R. Acclimation of photosynthesis to elevated CO2 in five C3 species [J]. Plant Physiology, 1989, 89: 590—596
[44] Drake B G, Gonzàlez-Meler M A, Long S P. More efficient plants: a consequence of rising atmospheric CO2 [J]. Annual Review of Plant Biology, 1997, 48: 609—639
[45] Boelen P, van de Poll W H, van der Strate H J, et al. Neither elevated nor reduced CO2 affects the photophysiological performance of the marine Antarctic diatom Chaetoceros brevis [J]. Journal of Experimental Marine Biology and Ecology, 2011, 406: 38—45
[46] Israel A, Hophy M. Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations [J]. Global Change Biology, 2002, 8(9): 831—840
[47] Tcherkez G G B, Farquhar G D, Andrews T J. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized [J]. Proceedings of the National Academy of Sciences, 2006, 103: 7246—7251
-
期刊类型引用(4)
1. 龙成凤,张达娟,王泽斌,张树林,毕相东,戴伟. 三种微藻对不同氮源去除率及CO_2固定效率的研究. 天津农学院学报. 2023(05): 34-39 .  百度学术
百度学术
2. 马丽,宋申,杨光智. 纤维素基多孔碳的制备及其CO_2吸附性能研究. 有色金属材料与工程. 2021(04): 27-32 .  百度学术
百度学术
3. 邢海亮,董训赞,韩本勇,耿树香,宁德鲁,马婷,余旭亚. 二氧化碳联合核桃壳提取液促进单针藻Monoraphidium sp.QLZ-3的生长和油脂积累. 化工进展. 2020(04): 1575-1582 .  百度学术
百度学术
4. 宋立荣,张琪,郑凌凌,李天丽,杜帆. 微藻种质资源库——藻类科学研究和产业发展的重要平台. 水生生物学报. 2020(05): 1020-1027 .  本站查看
本站查看
其他类型引用(3)





 下载:
下载: