SYNERGETIC EFFECT OF LIGHT AND NUTRIENTS ON THE RELEASE OF DISSLOVED ORGANIC CARBON FROM JUVENILES OF SACCHARINA JAPONICA
-
摘要: 文章以海带(Saccharina japonica)幼苗为实验材料, 对比了其在自然海水+50%海面光强、自然海水+100%海面光强、营养盐加富海水+50%海面光强、营养盐加富海水+100%海面光强4种条件下的溶解有机碳(DOC)释放速率, 以揭示光照与营养盐的协同作用对大型藻类释放DOC的影响机制。研究发现, 在自然海水条件下, 50%与100%海面光强照射下海带幼苗DOC释放速率分别为(11.67±3.07)和(22.65±4.58) μmol/(g·h), 随着光强增加, 释放DOC速率显著提升(P<0.05), 氧气净释放速率显著增大(P<0.05), 且二者光谱斜率差异显著(P<0.05); 在营养盐加富海水条件下, 50%与100%海面光强照射下海带幼苗DOC释放速率分别为(30.88±7.96)和(39.03±14.78) μmol/(g·h), 随着光强增加, 释放DOC速率提升不显著(P>0.05), 氧气净释放速率显著提高79.24%(P<0.05), 二者光谱斜率差异不显著(P>0.05)。结果表明, 在寡营养条件下(自然海水), 海带幼苗释放DOC速率与光照强度呈正相关, 表现为“溢出”机制; 在富营养条件下, 海带幼苗释放DOC速率与光照强度不相关, 表现为“扩散”机制。海带幼苗释放DOC同时受到“溢出”和“扩散”两种机制的调控, 何种机制占主导取决于营养水平。Abstract: Macroalgae farming is an essential part of offshore carbon sink. Large-scale macroalgae farming promotes the absorption of atmospheric CO2 by the ocean. As the primary producer of marine ecosystems, macroalgae can convert Dissolved Inorganic Carbon (DIC) into Organic Carbon (OC) through photosynthesis. Macroalgae can also release 20%—30% of photosynthesis products into seawater in the form of Dissolved Organic Carbon (DOC). According to estimates, the total amount of DOC released by macroalgae farming in China is about 822000 to 915000 tons, which can be transformed into 600000 tons of Recalcitrant Dissolved Organic Carbon (RDOC) every year through the action of Marine Microbial Carbon Pump (MCP). Therefore, seaweed culture plays a vital role in fisheries carbon sink. However, the synergistic effect of light and nutrients on DOC release by algae is still controversial. There are currently two hypotheses. One is the “overflow” hypothesis. Fogg believed that DOC release from algae was positively correlated with light intensity, which promoted the release of high molecular weight storage products. In Cherrier’s laboratory and field studies, the DOC release rate of planktonic algae was positively correlated with light intensity. After studying multiple experimental data on benthic algae, Barrón also found that the net DOC flux of benthic algae was positively correlated with light intensity. These reports show a significant correlation between light intensity and DOC release rate. Another hypothesis is “diffusion”. Bjornsen believes that the release rate of DOC from algae has nothing to do with light, but is closely related to the nutrient concentration. Nutrients promote the release of dissolved low molecules substances in algae. In Marañón’s algae culture experiment, there was no correlation between DOC release and light intensity. In the cross-experiment of light and nutrients carried out by Mueller, the correlation between DOC release of coral symbiotic algae and light disappeared when nutrients were added. The above studies show that light and nutrients are two critical environmental factors that regulate the release of DOC from algae. Saccharina japonica is the most important cluturing spcecies in China. According to statistics from the Food and Agriculture Organization of the United Nations (FAO), China had contributed 18% of the global S. japonica production in 2017.This study used juveniles of S. japonica as the research material and set up indoor crossover experiments based on the two factors of light and nutrient concentration. To explore the synergistic effect of light and nutrients on DOC release from macroalgae, we set up four experimental groups of natural sea water+50% sea surface light intensity, natural sea water+100% sea surface light intensity, and nutrient-enriched sea water+50% sea surface light intensity, nutrient-enriched sea water+100% sea surface light intensity, each group set up five parallel samples. Based on the above experimental conditions, the juveniles of S. japonica were cultured in a light incubator for 8h, and the water temperature was kept at (14±0.5)℃ during culture. The study found that under natural seawater conditions, the DOC rate of juveniles of S. japonica under 50% and 100% sea surface light intensity was (11.67±3.07) μmol/(g·h) and (22.65±4.58) μmol/(g·h), respectively. With the increase of light intensity, the DOC release rate was significantly improved (P<0.05), a substantial increased in the net oxygen release rate (P<0.05), and the difference of light spectrum slope was significant (P<0.05). Under the condition of nutrient-enriched seawater, the DOC release rate of juveniles of S. japonica under 50% and 100% sea surface light intensity was (30.88±7.96) μmol/(g·h) and (39.03±14.78) μmol/(g·h). With the increase of light intensity, the DOC release rate was not significant improved (P>0.05), the net oxygen release rate was significantly increased by 79.24% (P<0.05), and there was no significant difference in light spectrum slope (P>0.05).The results showed that under oligotrophic conditions (natural seawater), the DOC release rate of juveniles of S. japonica was positively correlated with the light intensity, indicating a “spillover” mechanism. Under eutrophic conditions (enriched seawater), the DOC release rate of juveniles of S. japonica was not related to the light intensity, which showed a “Diffusion” mechanism. The release of DOC from juveniles of S. japonica is regulated by two mechanisms, “spillover” and “diffusion”, which mechanism was dominant depends on the nutrient concentration.
-
海带(Saccharina japonica J.E.Areschoug)是褐藻纲海带属的一种藻类, 原产于东北亚地区(日本、朝鲜北部沿海和俄罗斯太平洋沿岸), 后来在我国辽东半岛和山东沿海地区广泛分布, 逐渐成为我国海水养殖规模最大的海藻物种。据世界粮农组织(FAO)的数据统计, 2017年我国贡献了全球海带产量的18%[1, 2]。目前, 我国养殖海带的主要品种包括“大连无边”“东方5号”“爱伦湾”和“901海带”等[3-5]。
近海大型藻类养殖碳汇是渔业碳汇的重要组成部分[6], 大型藻类的规模化养殖促进了海洋对大气CO2的吸收[7], 这一观点已经在不同海藻种类中得到证实。狐尾藻(Myriophyllum spicatum)在杭州湾的固碳效率为1.25 mg/(g DW∙h)[8], 龙须菜(Gracilaria lemaneiformis)在盐田湾的固碳效率最高达到了9.25 mg/(g DW·h)[9]。据估计我国每年养殖藻类固碳量约达3.52 Tg/Ca[10], 其中海带对碳汇渔业的贡献约占总养殖海藻的73%[11]。大型藻类作为海洋生态系统的初级生产者, 能够通过光合作用将海水中的溶解无机碳(Dissolved Inorganic Carbon, DIC)转化为有机碳, 并显著降低海水二氧化碳(CO2)分压, 促进了海洋对大气CO2吸收[12]。不仅如此, 大型海藻还能够将20%—30%光合作用产物以可溶性有机碳(Dissolved Organic Carbon, DOC)的形态释放到海水中[13]。据估算, 我国海藻养殖每年释放DOC总量约为(82.2—91.5)×107 kg, 海藻释放的DOC经海洋微型生物碳泵(Microbial Carbon Pump, MCP)作用, 每年可生成60×107 kg以上的惰性溶解有机碳(Recalcitrant Dissolved Organic Carbon, RDOC), 该碳汇量约为我国海岸带“蓝碳”埋藏量的1.7倍[10, 13]。由此可见, 海藻养殖在碳汇渔业中起到重要作用。
但关于光照与营养盐的协同作用对海藻释放DOC的影响机制仍存在争议。目前主要存在两种假说, 一种是“溢出”假说, Fogg[14]认为藻类释放DOC与光照强度呈正相关, 光强促进了溶解态大分子物质的释放。在Cherrier等[15]的室内和海区实验中, 浮游藻类的DOC释放速率均与光照强度呈正相关; BarrÃ3n等[16]对多个关于底栖藻类的实验数据研究后, 同样发现底栖海藻DOC净通量随光强增加而增大, 这些报道都表明光照强度与DOC释放速率有显著的相关性。另一种是“扩散”假说, Bjornsen[17]认为藻类释放DOC的速率与光照强度无关, 与营养盐含量密切相关, 营养盐促进了藻类中溶解态小分子物质的释放。在Marañón等[18]的藻类培养实验中, 没有发现DOC释放与光照强度有相关性, 且在Mueller等[19]开展的光照与营养盐的交叉实验中, 珊瑚共生藻DOC释放与光照强度的相关性在营养加富后消失。以上研究表明, 光照与营养盐是调节藻类释放DOC的两种重要环境因素。基于此, 本研究以海带幼苗为研究材料, 基于光照和营养盐浓度两个因素设置室内交叉实验, 探究光照与营养盐的协同作用对大型海藻释放DOC的影响机制, 以期为海藻增养殖提供理论与技术支持。
1. 材料与方法
1.1 试验材料
2020年12月从山东省荣成市东楮岛附近的海带养殖区(E 122°56′, N 37°05′)采集鲜活海带样品, 样品平均体长(34.26±8.99) cm, 平均湿重为(5.25±1.86) g。在实验开始前, 于室内循环水养殖系统中暂养5d, 暂养期间光合有效辐射(Photosynthetically Active Radiation, PAR)为(63±9) μmol photons/(m·s), 水温为(13.5±0.5)℃。
1.2 试验方法
设置了自然海水+50%海面光强、自然海水+100%海面光强、营养盐加富海水+50%海面光强、营养盐加富海水+100%海面光强4个实验组, 每组设置5个平行样。光照强度的设置参照曹昀等[20]和孙百晔等[21]的研究方法, 其中100% PAR和50% PAR分别为(102±5) 和(51±5) μmol photons/(m·s)。实验使用的自然海水中的磷酸盐、无机氮浓度分别为4.2和101 μg/L, 氮磷加富海水是在自然海水的基础上, 使用磷酸氢二钾、硝酸钾分析纯试剂分别配制成的100和1000 μg/L标准液进行加富, 使海水中营养盐的浓度达到藻类生长饱和浓度[22], 加富后各组氮、磷营养盐摩尔比分别为50%光强+自然海水(0.97﹕0.03)、100%光强+自然海水(0.92﹕0.06)、50%光强+氮磷加富(11.35﹕0.31)和100%光强+氮磷加富(11.42﹕0.81)。实验过程为从暂养的海带幼苗中挑选健康且规格相近的单株海带幼苗放入2 L经过酸洗的玻璃瓶中, 将海带幼苗按照上述4种不同的处理分别在光照培养箱中进行8h培养, 培养时水温保持在(14±0.5)℃。
1.3 海带幼苗释放DOC的速率检测
在培养开始和结束时, 分别取50 mL水样, 使用孔径0.45 μm滤膜进行抽滤, 抽滤后的水样于零下20℃冷藏柜(SC/SD-332)冷冻保存。使用岛津TOC-LCPH总有机碳分析仪测定样品DOC含量, 具体操作参照Mueller[19, 26]的方法。DOC释放速率[RDOC, μmol/(g·h)]指单位质量海带幼苗(干重)在单位时间内引起的水体DOC含量的变化, RDOC的计算公式为:
$ {R_{ {\rm{DOC}}}} = \frac{{({C_{{t}}}-{C_0}) \times V \times 1000}}{{{W_{\rm{D}}} \times {M_{\rm{C}}} \times t}} $
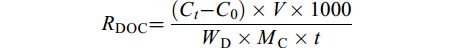
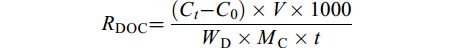
式中, Ct为实验组培养结束时的DOC浓度(mg/L), C0为对照组培养结束时的DOC浓度(mg/L), V为养殖用海水体积(L), WD为试验海带幼苗的干质量(kg), MC为碳的相对分子质量, t为试验处理时间(h)。
1.4 海带幼苗氧气释放速率检测
This page contains the following errors:
error on line 1 at column 1: Start tag expected, '<' not foundBelow is a rendering of the page up to the first error.
$ \Delta {C_{{{\rm{O}}_2}}} = \frac{{C{{(O)}_t}-C{{(O)}_0}}}{{{W_{\rm{D}}} \times h \times 2}} $
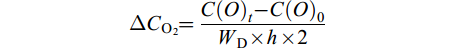
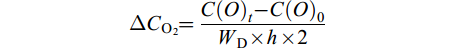
This page contains the following errors:
error on line 1 at column 1: Start tag expected, '<' not foundBelow is a rendering of the page up to the first error.
1.5 海带幼苗释放DOC占净初级生产力的比例检测
释放DOC占净初级生产力比重(Net Primary Productivity, NPP)比例(P, %), 是在假设碳固定与净产氧的摩尔比平衡(即1 mol碳固定等于1 mol氧气(O2)释放)条件下[19], 相同时间内海带幼苗释放DOC占净初级生产力比例。计算公式为:
$ P = \frac{{\Delta {C_{{\rm{DOC}}}}}}{{\Delta {C_{{{\rm{O}}_2}}}}} $
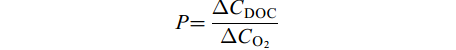
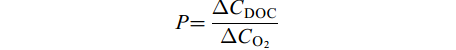
This page contains the following errors:
error on line 1 at column 1: Start tag expected, '<' not foundBelow is a rendering of the page up to the first error.
1.6 海带幼苗释放DOC的光谱斜率检测
在培养开始前和结束时, 分别取50 mL水样, 使用0.45 μm滤膜进行过滤, 抽滤后的水样于零下20℃冷藏柜(SC/SD-332)冷冻保存。使用UV-5100B紫外可见分光光度计测定水样的紫外可见吸收光谱, 具体操作参照陈昭宇等[24]的方法。吸收系数a(λ)的计算公式为:
$ a(\text{λ}) = \frac{{2.303 \times A(\text{λ} )}}{b} $
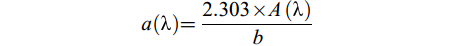
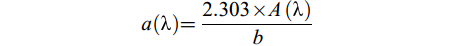
式中, A(λ)为吸光度, b为光程路径(m)。S275—295反映DOC相对分子质量与光反应活性, 相对分子质量越小值越大[25], 光谱斜率S的计算公式为:
$ a(\text{λ} ) = a({\text{λ} _0}) \times \exp [S \times ({\text{λ} _0}-\text{λ} )] $
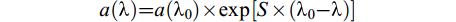
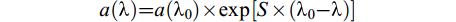
式中, a (λ)是DOM吸收系数(/m ), λ是波长(nm), λ0是参照波长(nm)。
1.7 数据分析
实验数据通过Excel 2019进行整理, 使用SPSS 26. 0进行单因素方差分析, 差异显著水平设置为P<0.05, 用LSD法进行多重比较, 图中不同字母表示差异水平为P<0.05, 相同字母表示差异水平为P>0.05, 使用OriginLab OriginPro 2021b SR1 v9.8.5.204进行作图。
2. 结果
2.1 不同实验条件下海带幼苗的DOC释放速率
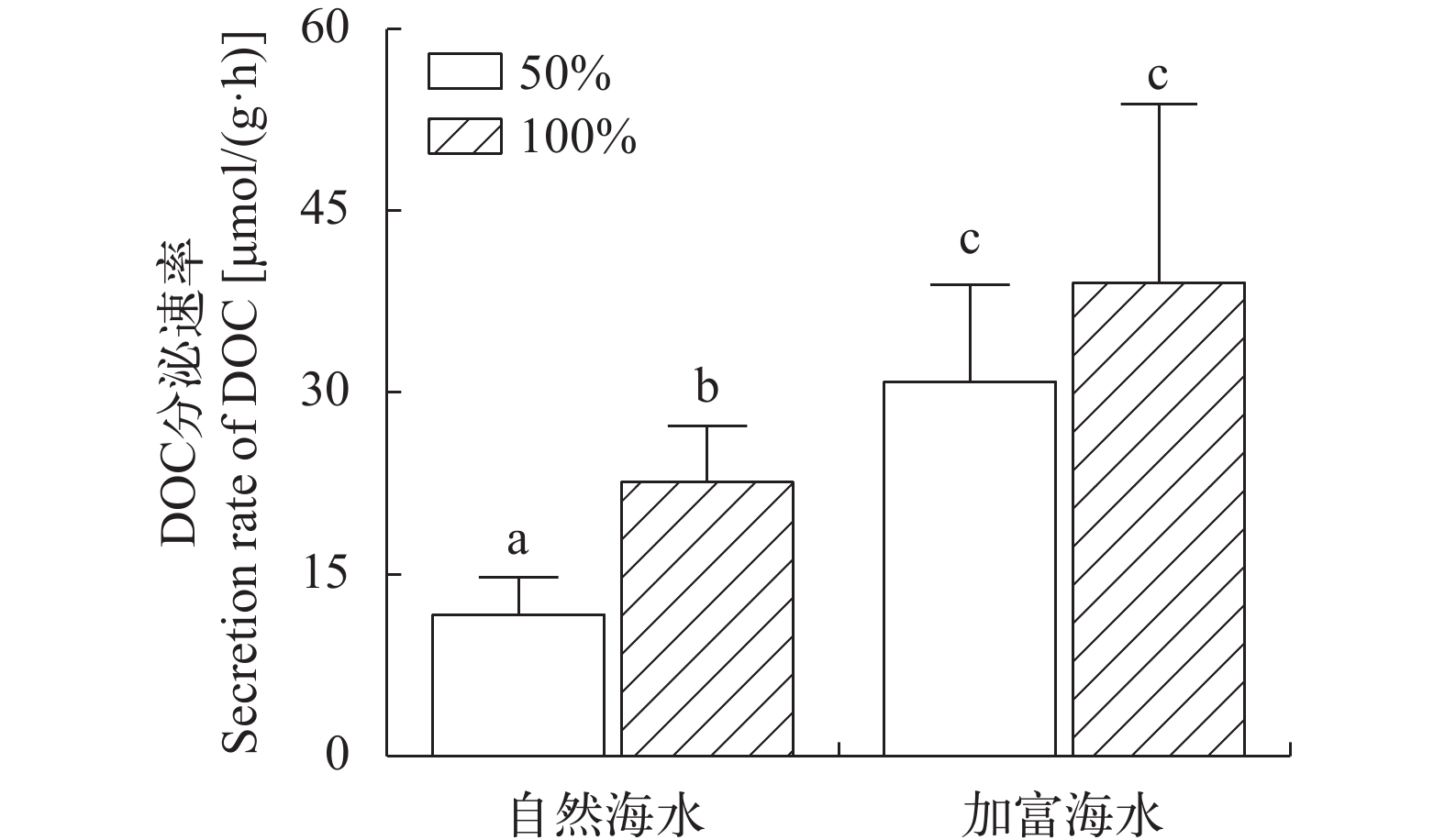
在自然海水条件下, 100%光强条件下海带幼苗DOC释放速率显著高于50%光强条件(P<0.05), 表明此条件下光强与海带幼苗释放DOC速率呈正相关性。在加富海水条件下, 100%光强与50%光强释放DOC速率差异不显著(P>0.05), 表明此条件下光强与海带幼苗释放DOC无相关性。但在同等光照强度条件下, 加富海水组释放DOC速率显著高于自然海水组(P<0.05), 表明营养加富显著提升了海带幼苗DOC释放速率(图 1)。
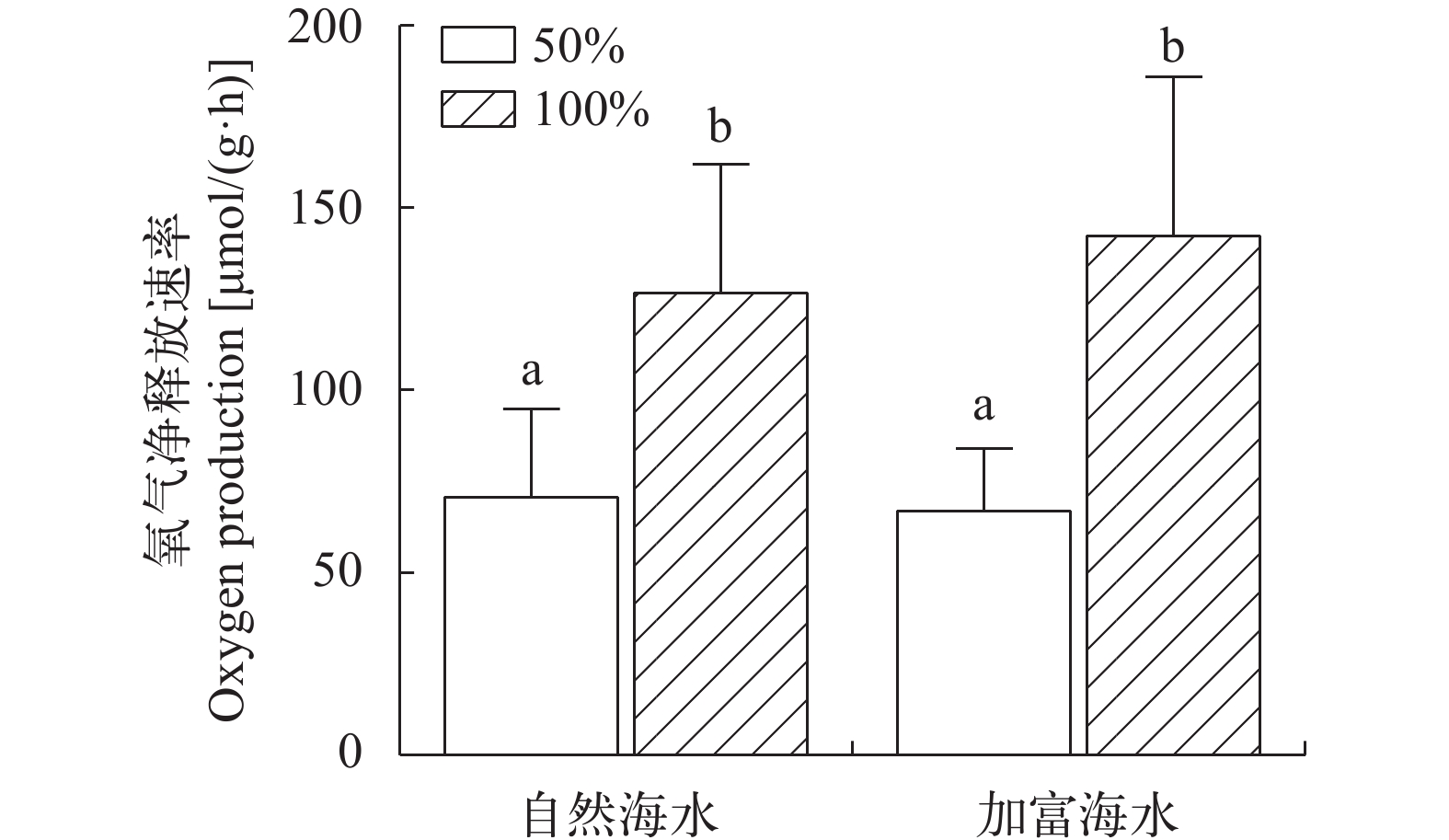
![]() 图 1 不同实验条件下海带幼苗DOC释放速率平均数后上标相同代表组间差异不显著(P>0.05), 平均数后上标不同代表组间有显著性差异(P>0.05), 下同Figure 1. DOC secretion rate of juveniles of S. japonica under different experimental conditionsTreatment combinations with the same letter are not significantly different at P>0.05, treatment combinations with the same letter are not significantly different at P>0.05, the same applies below
图 1 不同实验条件下海带幼苗DOC释放速率平均数后上标相同代表组间差异不显著(P>0.05), 平均数后上标不同代表组间有显著性差异(P>0.05), 下同Figure 1. DOC secretion rate of juveniles of S. japonica under different experimental conditionsTreatment combinations with the same letter are not significantly different at P>0.05, treatment combinations with the same letter are not significantly different at P>0.05, the same applies below2.2 不同实验条件下的海带幼苗释放DOC的光谱斜率
在自然海水条件下, 100%光强S275—295显著低于50%光强S275—295(P<0.05), 表明此条件下随着光强增加, 海带幼苗释放的DOC相对分子量增大(表 1)。但在加富海水条件下, 100%光强S275—295与50%光强S275—295差异不显著(P>0.05), 表明此条件下光照强度并没有改变海带幼苗释放DOC的相对分子量。加富条件下的海带幼苗在100%光强时S275—295显著高于自然海水条件下100%光强S275—295(P<0.05), 表明在同等光照条件下, 营养盐加富显著降低了海带幼苗释放DOC的相对分子量(表 1)。
表 1 不同实验条件下海带幼苗释放DOC的S275—295值Table 1. S275—295 values of DOC released from juveniles of S. japonica under different experimental conditions实验条件
Experimental condition均值
Mean value
(S275—295, nm)范围
Range
(S275—295, nm)自然海水
Natural sea water50% 0.01834±
0.00239 a0.01614—
0.02088100% 0.01045±
0.00118 b0.00981—
0.01181加富海水
Nutrient-enriched sea water50% 0.01522±
0.00186 ac0.01375—
0.01731100% 0.01382±
0.00120 c0.01244—
0.01463注: 平均数后上标相同代表组间差异不显著(P>0.05), 平均数后上标不同代表组间有显著性差异(P>0.05)Note: Treatment combinations with the same letter are not significantly different at P>0.05, treatment combinations with the same letter are not significantly different at P>0.05 2.3 不同实验条件下海带幼苗氧气释放速率
在自然海水、加富条件下, 100%光强组氧气释放速率均显著高于50%光强组(P<0.05), 表明海带幼苗不管处于何种营养水平, 光照强度提升均能显著促进氧气释放。但在同等光照水平下(100%光强、50%光强), 营养盐加富组氧气释放速率与自然海水组差异不显著(P>0.05), 表明海带幼苗不管处于何种光照水平, 营养盐加富未能显著促进氧气释放(图 2)。
2.4 不同实验条件下海带幼苗释放DOC占净初级生产力(NPP)比重
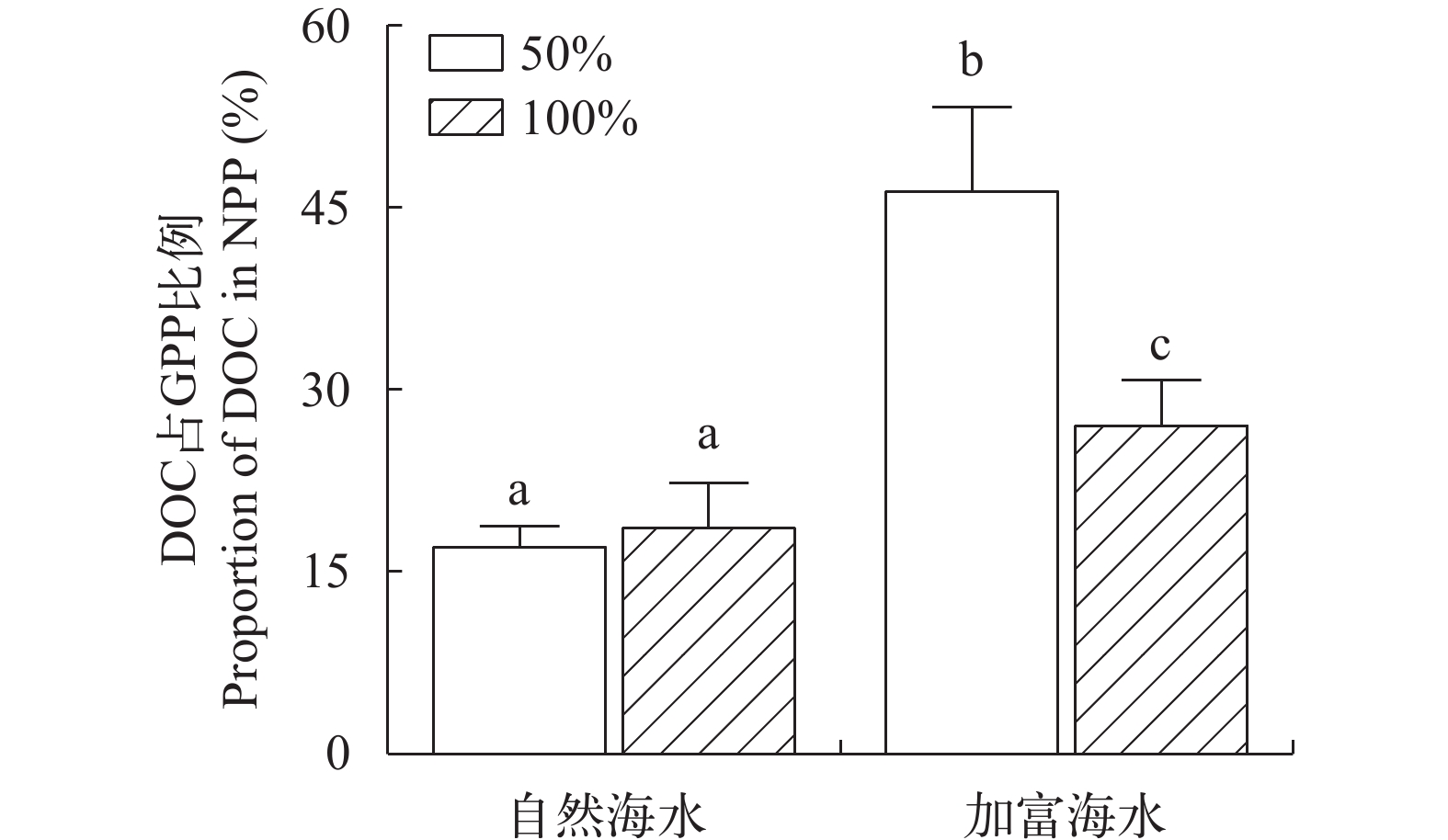
在自然海水条件下, 100%光强组与50%光强组释放DOC占NPP比例差异不显著(P>0.05), 表明在此条件下, 光照提升并未显著改变海带幼苗的DOC净释放率。在加富条件下, 50%光强组释放DOC占NPP比例显著高于100%光强组(P<0.05), 表明在此条件下, 光照强度提升反而降低了海带幼苗的DOC净释放率。在同等光照强度水平下(100%光强、50%光强), 加富组释放DOC占NPP比例均显著高于自然海水组(P<0.05), 表明通过营养盐加富促进了海带幼苗的DOC净释放率(图 3)。
3. 讨论
尽管光照与营养盐的协同作用对藻类释放DOC的影响机制尚不明确, 但似乎“溢出”“扩散”两种假说均不足以解释其全部过程, 各有大量研究结果支持某一项“假说”也间接说明了这一点。如在Mueller等[19]的光照与营养盐交叉实验中发现, 在寡营养盐条件下, 珊瑚藻(Coralline algae)释放DOC与光强具有正相关性, “溢出”机制起作用; 但在添加氮磷营养盐后, 释放DOC与光强差异不显著, 表明在营养充足的条件下, “扩散”机制起作用。结合已有研究结果, 我们发现往往是在寡营养条件下, DOC释放与光照强度呈正相关, 表现为“溢出”机制。如前人对浮游藻类、珊瑚藻(Coralline algae)和巨藻(Macrocystis pyrifera)等藻类的研究发现, 当藻体处在营养盐浓度较低的自然海水中时, DOC释放随光强的增大而升高, 与“溢出”机制相符[15, 26, 27]。而在营养充足的条件时, “溢出”机制消失, 表现为“扩散”机制占主导。在Marañón[18]的研究中, 富营养条件下浮游藻类的DOC释放与光强没有显著相关性, 在Wyatt[22]对刚毛藻[Cladophora glomerata (L.) Kütz.]的研究中也有相同现象。为此有研究提出, 很有可能“溢出”“扩散”2种机制均存在, 只是在特定条件下某项机制占主导。在本研究中, 我们发现在自然海水(寡营养)条件下, 海带幼苗释放DOC与光照强度呈正相关, 且释放DOC相对分子量也随光强上升而提高, 表明在寡营养条件下, 海带幼苗释放DOC受“溢出”机制调节(图 1)。在加富条件下, 海带幼苗释放DOC与光强无相关性, 表明“溢出”机制消失; 但在同等光照强度条件下, 加富组释放DOC速率显著高于自然海水组(图 1), 不仅如此, 加富还显著提升了DOC净释放率(图 2), 且加富显著降低了释放DOC相对分子量(表 1), 表明“扩散”机制占主导。
本研究推测海带幼苗释放DOC过程中“溢出”“扩散”机制均存在。在寡营养条件下, “溢出”机制占主导; 而营养盐水平一旦超过藻类生长饱和浓度, 此时“溢出”机制消失, “扩散”机制占主导。研究推测, 藻类光合作用受光照强度调节, 而细胞的生长则受无机营养盐限制。在N、P营养盐限制条件下, 随着光照强度升高, 藻类细胞光合作用产出将超过细胞生长对有机质的需求。此时细胞光合固定有机碳的速率将超过N、P供给速率, 从而导致细胞内C元素大量富集, 细胞内C﹕N﹕P比上升, 生成的有机质主要以高分子量物质为主。此时藻类表现为光强的增加促进有机质生成, 从而加剧DOC的释放, “溢出”机制占主导。当环境中N、P营养盐富集时, 此时藻类细胞内C﹕N﹕P比下降, 因此生成的有机质主要以低分子量物质为主。低分子量的DOC跨膜运输受细胞膜内外浓度差控制, 表现为“扩散”机制占主导。
本研究发现, 海带幼苗在100%光强+氮磷加富条件下DOC释放速率最高(图 1)。为提升养殖海带碳汇效率, 可适当调整养殖模式, 通过同时提升海区营养盐及光照强度以促进养殖海带DOC的释放。海带养殖区往往营养盐含量较低, 呈现氮磷营养盐限制的状态, 为促进海带释放DOC, 可在海带养殖区进行N、P营养盐的缓释。虽然大型海藻对赤潮微藻生长具有一定的抑制作用[28, 29], 但也应注意N、P营养盐的缓释可能引发微藻暴发性增殖造成的赤潮风险。在进行营养盐缓释的同时, 适当降低海带养殖密度, 使海带叶片在单位面积上能够接收到更多光照。房景辉等[30, 31]的研究表明, 降低养殖密度的标准化养殖同时也能提升养殖海带品质与产量。本研究可以为养殖海带增汇提供一定的理论依据与技术参考。
-
图 1 不同实验条件下海带幼苗DOC释放速率
平均数后上标相同代表组间差异不显著(P>0.05), 平均数后上标不同代表组间有显著性差异(P>0.05), 下同
Figure 1. DOC secretion rate of juveniles of S. japonica under different experimental conditions
Treatment combinations with the same letter are not significantly different at P>0.05, treatment combinations with the same letter are not significantly different at P>0.05, the same applies below
表 1 不同实验条件下海带幼苗释放DOC的S275—295值
Table 1 S275—295 values of DOC released from juveniles of S. japonica under different experimental conditions
实验条件
Experimental condition均值
Mean value
(S275—295, nm)范围
Range
(S275—295, nm)自然海水
Natural sea water50% 0.01834±
0.00239 a0.01614—
0.02088100% 0.01045±
0.00118 b0.00981—
0.01181加富海水
Nutrient-enriched sea water50% 0.01522±
0.00186 ac0.01375—
0.01731100% 0.01382±
0.00120 c0.01244—
0.01463注: 平均数后上标相同代表组间差异不显著(P>0.05), 平均数后上标不同代表组间有显著性差异(P>0.05)Note: Treatment combinations with the same letter are not significantly different at P>0.05, treatment combinations with the same letter are not significantly different at P>0.05 -
[1] 孙琰晴. 无机砷在海带中的富集转化及其毒理效应 [D]. 上海: 上海海洋大学, 2020: 1-3. Sun Y Q. Bioaccumulation and transformation of inorganic arsenic in Laminaria japonica and toxicological effect [D]. Shanghai: Shanghai Ocean University, 2020: 1-3.
[2] 郭云峰, 赵文武. 中国渔业统计年鉴 [M]. 北京: 中国农业出版社, 2017: 17-46. Guo Y F, Zhao W W. China Fishery Statistical Yearbook [M]. Beijing: China Agriculture Press, 2017: 17-46.
[3] 袁廷柱, 刘凯, 詹冬梅, 等. 长岛海区海带品种优选试验 [J]. 河北渔业, 2020(10): 50-52,63. doi: 10.3969/j.issn.1004-6755.2020.10.012 Yuan T Z, Liu K, Zhan D M, et al. Optimization of Saccharina japonica varieties in Changdao Sea area [J]. Hebei Fisheries, 2020(10): 50-52,63. doi: 10.3969/j.issn.1004-6755.2020.10.012
[4] 王珊珊, 张瑞标, 常丽荣, 等. 威海桑沟湾海域不同海带品种/系的生长状况研究 [J]. 大连海洋大学学报, 2020, 35(1): 96-102. Wang S S, Zhang R B, Chang L R, et al. Growth performance of different varieties/lines of kelp Saccharina japonica in Sanggou Bay, Weihai, China [J]. Journal of Dalian Ocean University, 2020, 35(1): 96-102.
[5] 王国文. 我国主要海带栽培品种的性状评价与遗传学分析 [D]. 北京: 中国农业科学院, 2009: 4-7. Wang G W. Traits evaluation and genetic analysis of main cultivars of Laminaria in China [D]. Beijing: Chinese Academy of Agricultural Sciences Dissertation, 2009: 4-7.
[6] 唐启升, 刘慧. 海洋渔业碳汇及其扩增战略 [J]. 中国工程科学, 2016, 18(3): 68-73. doi: 10.3969/j.issn.1009-1742.2016.03.012 Tang Q S, Liu H. Strategy for carbon sink and its amplification in marine fisheries [J]. Engineering Sciences, 2016, 18(3): 68-73. doi: 10.3969/j.issn.1009-1742.2016.03.012
[7] 蒋增杰, 方建光, 韩婷婷, 等. 大型藻类规模化养殖水域海-气界面CO2交换通量估算 [J]. 渔业科学进展, 2013, 34(1): 50-56. doi: 10.3969/j.issn.1000-7075.2013.01.008 Jiang Z J, Fang J G, Han T T, et al. Estimation of sea-air CO2 flux in seaweed aquaculture area, Lidao Bay [J]. Progress in Fishery Sciences, 2013, 34(1): 50-56. doi: 10.3969/j.issn.1000-7075.2013.01.008
[8] Bao Y, Huo Y, Duan Y, et al. Growth and nutrient uptake of Myriophyllum spicatum under different nutrient conditions and its potential ecosystem services in an enclosed sea area in the East China Sea [J]. Marine Pollution Bulletin, 2020(151): 110801. doi: 10.1016/j.marpolbul.2019.110801
[9] Duan Y, Yang N, Hu M, et al. Growth and nutrient uptake of Gracilaria lemaneiformis under different nutrient conditions with implications for ecosystem services: a case study in the laboratory and in an enclosed mariculture area in the East China Sea [J]. Aquatic Botany, 2019(153): 73-80. doi: 10.1016/j.aquabot.2018.11.012
[10] 张永雨, 张继红, 梁彦韬, 等. 中国近海养殖环境碳汇形成过程与机制 [J]. 中国科学: 地球科学, 2017, 47(12): 1414-1424. doi: 10.1360/N072017-00344 Zhang Y Y, Zhang J H, Liang Y T, et al. Carbon sequestration processes and mechanisms in coastal mariculture environments in China [J]. Scientia Sinica (Terrae)
, 2017, 47(12): 1414-1424. doi: 10.1360/N072017-00344 [11] 纪建悦, 王萍萍. 我国海水养殖业碳汇能力测度及其影响因素分解研究 [J]. 海洋环境科学, 2015, 34(6): 871-878. Ji J Y, Wang P P. Research on China’s mariculture carbon sink capacity and influencing factors [J]. Marine Environmental Science, 2015, 34(6): 871-878.
[12] Li J, Murauchi Y, Ichinomiya M. Seasonal changes in photosynthesis and nutrient uptake in Laminaria japonica [J]. Aquaculture Science, 2007, 55(4): 587-597.
[13] 焦念志, 梁彦韬, 张永雨, 等. 中国海及邻近区域碳库与通量综合分析 [J]. 中国科学: 地球科学, 2018, 48(11): 1393-1421. doi: 10.1360/N072018-00014 Jiao N Z, Liang Y T, Zhang Y Y, et al. Carbon pools and fluxes in the China Seas and adjacent oceans [J]. Scientia Sinica (Terrae)
, 2018, 48(11): 1393-1421. doi: 10.1360/N072018-00014 [14] Fogg G E. The ecological significance of extracellular products of phytoplankton photosynthesis [J]. Botanica Marina, 1983, 26(1): 3-14.
[15] Cherrier J, Valentine S, Hamill B, et al. Light-mediated release of dissolved organic carbon by phytoplankton [J]. Journal of Marine Systems, 2015(147): 45-51. doi: 10.1016/j.jmarsys.2014.02.008
[16] BarrÃ3n C, Apostolaki E T, Duarte C M. Dissolved organic carbon fluxes by seagrass meadows and macroalgal beds [J]. Frontiers in Marine Science, 2014(1): 42-1.
[17] Bjørrisen P K. Phytoplankton exudation of organic matter: Why do healthy cells do it [J]? Limnology and Oceanography, 1988, 33(1): 151-154.
[18] Marañón E, Cermeño P, Pérez V. Continuity in the photosynthetic production of dissolved organic carbon from eutrophic to oligotrophic waters [J]. Marine Ecology Progress Series, 2005(299): 7-17. doi: 10.3354/meps299007
[19] Mueller B, den Haan J, Visser P M, et al. Effect of light and nutrient availability on the release of dissolved organic carbon (DOC) by Caribbean turf algae [J]. Scientific Reports, 2016, 6(1): 1-9. doi: 10.1038/s41598-016-0001-8
[20] 曹昀, 罗姗姗, 陈冰祥. 光强对菹草生长及抗氧化酶活性的影响 [J]. 水生生物学报, 2018, 42(4): 846-853. doi: 10.7541/2018.104 Cao Y, Luo S S, Chen B X. Effects of light intensity on growth and antioxidant enzyme activity of Potamogeton crispus [J]. Acta Hydrobiologica Sinica, 2018, 42(4): 846-853. doi: 10.7541/2018.104
[21] 孙百晔, 梁生康, 王长友, 等. 光照与东海近海中肋骨条藻(Skeletonema costatum)赤潮发生季节的关系 [J]. 环境科学, 2008, 29(7): 1849-1854. doi: 10.3321/j.issn:0250-3301.2008.07.015 Sun B Y, Liang S K, Wang C Y, et al. Role of irradiance on the seasonality of Skeletonema costatum cleve blooms in the coastal area in East China Sea [J]. Environmental Science, 2008, 29(7): 1849-1854. doi: 10.3321/j.issn:0250-3301.2008.07.015
[22] Wyatt K H, Tellez E, Woodke R L, et al. Effects of nutrient limitation on the release and use of dissolved organic carbon from benthic algae in Lake Michigan [J]. Freshwater Science, 2014, 33(2): 557-567. doi: 10.1086/675453
[23] 权伟, 应苗苗, 王怡娟, 等. 不同光环境下两种品系坛紫菜DOC分泌特性研究 [J]. 大连海洋大学学报, 2018, 33(3): 300-306. Quan W, Ying M M, Wang Y J, et al. Secretion characteristics of dissolved organic carbon (DOC) in two strains of laver Porphyry haifanesis under different light conditions [J]. Journal of Dalian Ocean University, 2018, 33(3): 300-306.
[24] 陈昭宇, 李思悦. 三峡库区城镇化背景下河流DOM的吸收及荧光光谱特征 [J]. 环境科学, 2019, 40(12): 5309-5317. Chen Z Y, Li S Y. Absorption and fluorescence spectra of dissolved organic matter in rivers of the Three Gorges Reservoir area under the background of urbanization [J]. Environmental Science, 2019, 40(12): 5309-5317.
[25] Wada S, Aoki M N, Tsuchiya Y, et al. Quantitative and qualitative analyses of dissolved organic matter released from Ecklonia cava Kjellman, in Oura Bay, Shimoda, Izu Peninsula, Japan [J]. Journal of Experimental Marine Biology and Ecology, 2007, 349(2): 344-358. doi: 10.1016/j.jembe.2007.05.024
[26] Mueller B, van der Zande R M, van Leent P, et al. Effect of light availability on dissolved organic carbon release by Caribbean reef algae and corals [J]. Bulletin of Marine Science, 2014, 90(3): 875-893. doi: 10.5343/bms.2013.1062
[27] Reed D C, Carlson C A, Halewood E R, et al. Patterns and controls of reef-scale production of dissolved organic carbon by giant kelp Macrocystis pyrifera [J]. Limnology and Oceanography, 2015, 60(6): 1996-2008. doi: 10.1002/lno.10154
[28] 王悠, 俞志明, 宋秀贤, 等. 大型海藻与赤潮微藻以及赤潮微藻之间的相互作用研究 [J]. 环境科学, 2006, 27(2): 274-280. doi: 10.3321/j.issn:0250-3301.2006.02.015 Wang Y, Yu Z M, Song X X, et al. Effects of macroalgae on growth of 2species of bloom microalgae and interactions between these microalgae in laboratory culture [J]. Environmental Science, 2006, 27(2): 274-280. doi: 10.3321/j.issn:0250-3301.2006.02.015
[29] 安蓁. 大型海藻及纳米材料对赤潮中肋骨条藻的抑制作用研究 [D]. 青岛: 中国海洋大学, 2008: 22-29. An Z. The research of inhibitory effect on red tide Skeletonema costatum by macroalgae and nanomaterials [D]. Qingdao: Ocean University of China, 2008: 22-29.
[30] 房景辉, 何为, 毛玉泽, 等. 桑沟湾标准化与传统养殖模式海带的营养成分比较 [J]. 渔业科学进展, 2021, 42(2): 170-175. Fang J H, He W, Mao Y Z, et al. A comparison of nutrients in kelp cultured in standardized and traditional long-line modes in Sanggou Bay [J]. Progress in Fishery Sciences, 2021, 42(2): 170-175.
[31] 房景辉, 蒋增杰, 蔺凡, 等. 桑沟湾海带标准化养殖模式的优势探析 [J]. 渔业科学进展, 2020, 41(5): 134-140. Fang J H, Jiang Z J, Lin F, et al. Analysis on the advantages of standard kelp long line culture in Sanggou Bay [J]. Progress in Fishery Sciences, 2020, 41(5): 134-140.
-
期刊类型引用(1)
1. 艾晓寒,涂晓杰,许萍萍,夏亦雪,毕永红. 汞胁迫下雪衣藻的生理活性及其藻源性有机物分析. 生态毒理学报. 2023(06): 245-256 .  百度学术
百度学术
其他类型引用(2)



 下载:
下载: