HIGH AMMONIA EXPOSURE AFTER PRE- ACCLIMATIZATION TO LOW AMMONIA CONCENTRATION ON GILL TISSUE STRUCTURE, IMMUNE INDICES, AND RELATED GENES IN TACHYSURUS FULVIDRACO
-
摘要:
研究旨在研究低浓度氨氮预适应后高浓度氨氮暴露对黄颡鱼(Tachysurus fulvidraco)鳃组织结构、免疫指标及相关基因的影响。将黄颡鱼[体重: (3.13±0.49) g]分别暴露于低浓度氨氮(4.7 mg/L, 10%96h LC50)中预先适应3d、7d、14d后, 在高浓度氨氮(23.7 mg/L, 50%96h LC50)暴露12h和48h。结果显示: (1)在高浓度氨氮胁迫48h后, 不同时长低浓度氨氮预适应组(3d、7d、14d)和对照组(未经过低浓度氨氮预适应)鳃组织细胞出现空泡化, 鳃小片变短、卷曲、合并等不同程度的病理变化; 但低浓度氨氮预适应组的鳃组织在高浓度氨氮暴露12h和48h时损伤程度均低于对照组, 且预适应14d组在高浓度氨氮暴露12h和48h时鱼的鳃组织损伤程度最小。(2)在高浓度氨氮胁迫48h后, 预适应组和对照组的黄颡鱼体内LZM活性和IgM、C3、C4含量呈现一定的升高; 所有预适应组的LZM活性和IgM、C3含量显著升高, C4含量则只有预适应7d和14d显著升高。高浓度氨氮胁迫12h时, 预适应14d组的LZM活性和C3、C4含量均显著高于其他组(P<0.05)。(3)在高浓度氨氮胁迫12h时, tnf-α和c3基因表达水平显著下调; il-1β基因表达水平无显著变化; 仅预适应7d组 igm基因表达水平的显著上调。上述结果表明, 经过低浓度氨氮预适应后高浓度氨氮暴露, 黄颡鱼鳃组织损伤程度降低, LZM活性和IgM、C3、C4含量升高, 黄颡鱼在低浓度氨氮预适应后对高浓度氨氮环境具有一定的耐受能力。
Abstract:Ammonia nitrogen is an important water quality indicator in fish culture, as concentrations exceeding the safe range can cause stress to fish. The study aimed to investigate the effects of high ammonia exposure on the gill tissue structure, immune indexes, and related genes of yellow catfish (Tachysurus fulvidraco) after pre-acclimatization with low ammonia concentration. Yellow catfish [body weight: (3.13±0.49) g] were exposed to low concentration of ammonia nitrogen (4.7 mg/L, 10%96h LC50) after pre-acclimated for 3, 7, and 14d, followed by exposure to high ammonia nitrogen concentrations (23.7 mg/L, 50% 96h LC50) for 12h and 48h, respectively. The results showed that: (1) after 48h of high ammonia nitrogen stress, the gill structure of yellow catfish displayed various pathological changes such as vacuolization, shortening, curling, and merging of gill lamellae. However, the pre-acclimated groups (3d, 7d, and 14d) experienced less damage compared to the control group (not pre-acclimated to the low concentration of ammonia nitrogen). The control group showed different pathological changes such as vacuolization, gill lamellae shortening, curling, and merging.In contrast, the gill tissues of the pre-acclimated group were less damaged at both 12h and 48h of exposure to the high concentration of ammonia nitrogen. Specifically, the 14d pre-acclimated group exhibited the least gill tissue damage at these time points. (2) After 48h of high-concentration ammonia nitrogen stress, the LZM activity and IgM, C3 and C4 contents of yellow catfish increased to varying degrees in both pre-adapted and control groups. In all pre-adapted groups, the LZM activity and IgM, C3 contents showed significantly increased, while C4 contents were only significantly increased only in the pre-adapted 7d and the 14d group. At 12h of high-concentration ammonia nitrogen stress, the LZM activity and C3, C4 contents in the 14d group were significantly increased. Additionally, the LZM activity and C3, C4 contents of the 14d group were significantly increased. C3 and C4 contents were significantly higher than those of other groups at 12h of high concentration ammonia stress (P<0.05). (3) The expression levels of tnf-α and c3 genes were significantly down-regulated at 12h of high ammonia nitrogen stress, while in the expression level of il-1β gene showed no significant change. The expression level of igm gene was significantly up-regulated only in the 7d pre-acclimatization group. The above results indicated that pre-acclimatization to low concentrations of ammonia nitrogen, followed by exposure to high concentration of ammonia nitrogen, resulted in reduced gill tissue damage, increased LZM activity, and elevated IgM, C3 and C4 contents in yellow catfish. This suggests that yellow catifish develop a certain tolerance to high concentrations of ammonia nitrogen after pre-acclimatization to low concentration of ammonia nitrogen.
-
Keywords:
- Pre-acclimatization /
- Ammonia nitrogen stress /
- Gills /
- Immunity /
- Tachysurus fulvidraco
-
生物长期暴露于有毒物中适应后, 对暴露于致死浓度有毒物耐受性增强[1], 越来越多的证据表明, 预先暴露于非致死浓度的有毒物可提高鱼类对其的耐受性。例如, 鲤(Cyprinus carpio)低氨预适应后会激活其在高氨环境的耐受性, 且延长在高氨环境中的存活时间, 这可能是鱼体通过一系列生理生化、转录等过程预先启动了相关适应机制, 从而促进生物体快速地应对更高的环境压力[2]。预适应不同温度对草鱼(Ctenopharyngodon idella)的耐热性有影响, 提高预适应的温度可以激活适应途径并增加草鱼的耐热性[3]。大黄鱼(Pseudosciaena crocea)通过预适应低浓度铜提高脾脏和头肾免疫反应, 减轻高浓度铜诱导的免疫毒性[4]; 鳉(Heterandria formosa)预适应低浓度铜可延长其在致死铜浓度的存活时间[5]。这些研究表明, 鱼类在面临环境压力时预适应的重要意义。
池塘养殖过程常存在超负荷养殖, 饲料投喂过量, 氨氮循环失调等问题, 导致养殖鱼类反复处于氨氮胁迫状态。水体中的氨氮以NH3和${\rm{NH}}^+_4 $两种形式存在, 由于前者不带电荷, 具有脂溶性极易穿透鳃细胞膜, 更具有毒害作用[6]。在机体水平上, 氨氮会导致鱼类换气过度、过度兴奋、昏迷和抽搐; 在细胞水平上, 氨氮可以通过损害鱼体的三羧酸循环来干扰其能量代谢[7]。已有研究表明, 暴露在高浓度氨氮环境中会抑制鱼类的生长, 造成免疫、代谢功能紊乱, 最终造成鳃、肠道、肝脏组织损伤[8—11]。在硬骨鱼中, 鳃的每个鳃弓支撑着多根鳃丝, 鳃丝上有许多鳃小片, 这些鳃小片是气体交换的主要场所, 氨氮暴露会引起鳃组织病变, 出现鳃丝肿胀增生, 黏液增厚, 鳃小片呼吸上皮肿胀等现象[12], 影响鱼类的生理功能。鱼类免疫系统包括适应性免疫和非特异性免疫。史氏鲟(Acipenser schrenckii)幼鱼在慢性氨氮胁迫后, 鱼体LZM 活性及 IgM 含量均表现为逐渐降低的趋势, 表明慢性氨氮胁迫降低了 LZM 活性及IgM 的合成, 抑制了鱼体的免疫功能, 降低了鱼体的免疫力[13]。鳙(Aristichthys nobilis)随着氨浓度和暴露时间的增加, 肿瘤坏死因子(TNF-α)、白细胞介素6 (IL-6)、白细胞介素12 (IL-12)、白细胞介素1β (IL-1β)的mRNA表达显著增强, 抗炎因子白细胞介素10 (IL-10)的表达受到抑制, 氨暴露会干扰鱼类免疫反应并引起炎症[14]。芙蓉鲤鲫(Cyprinus capio furong.♀×Carassius auratus red var.♂)在氨氮胁迫后ACP和AKP活性出现不同程度的增强, 表明氨氮激活了磷酸酶介导的非特异性免疫系统[15]。鳃是鱼类的免疫组织, 因其直接与外界环境接触, 是抵御各种环境压力的第一道防线, 有证据表明, 氨氮胁迫可诱导氧化应激, 破坏防御系统的平衡, 损伤免疫系统并引起鱼的免疫反应[16]。鱼类在预适应低氨氮环境所产生的免疫反应是否会在机体内形成印记, 从而刺激鱼体对高氨氮暴露进行更快的保护性调整, 有待进一步研究。
黄颡鱼(Tachysurus fulvidraco)隶属鲶形目(Siluriformes)鲿科(Bagridae)黄颡鱼属(Pelteobagrusc Bleeker), 是我国重要的养殖经济鱼类之一, 分布于我国长江和珠江流域自然水体。黄颡鱼是典型的底栖无鳞鱼类, 对外界环境因子变化具有较高的敏感响应, 已被筛选为环境污染物毒性效应研究的理想实验鱼[17]。本研究旨在综合分析低浓度氨氮预适应后高浓度氨氮暴露对黄颡鱼的组织结构、免疫指标及相关基因的影响。本研究将对黄颡鱼养殖具有实际指导意义, 并提供低浓度氨氮预适应对后期氨氮胁迫的免疫分子机制理论基础。
1. 材料与方法
1.1 实验鱼
实验用黄颡鱼(3.13±0.49) g均为淮安市水生生物研究中心自行繁殖的同批次鱼苗,在实验室中暂养2周。暂养期间水温(25.5±1.0)℃,每天早晚(8:30和17:30)投喂2次商品配合饲料,日投喂量为鱼体质量的3%—5%,每天下午换水30%。在暂养结束后, 选择规格一致的健康黄颡鱼幼鱼随机分配于12个90 L的水族箱中, 每个水族箱中养殖45尾并放置1个充气石。实验容器为60 cm×45 cm×45 cm的水族箱。实验用水为曝气48h以上的自来水, 实验溶液首先用NH4Cl (分析纯)配制成10g/L的母液,实验时按比例稀释至所需浓度。
1.2 实验设计
在暂养2周后, 本研究参考课题组之前的研究得知黄颡鱼幼鱼96h半数致死浓度[18], 将实验鱼暴露于低浓度氨氮(4.7 mg/L, 10%96h LC50)中预适应3d、7d和14d, 将经过不同时长低浓度氨氮预适应组(3d、7d、14d)和对照组(未经过低浓度氨氮预适应)的实验鱼分别暴露于高浓度氨氮(23.7 mg/L, 50%96h LC50)中12h和48h。每组设3个平行, 通过氨氮胁迫的方法对黄颡鱼幼鱼进行不同时长的低浓度氨氮预适应后高浓度氨氮实验。仅在低浓度氨氮预适应实验期间投喂, 每天早晚(8:30和17:30)投喂2次商品配合饲料, 每次投喂1h后捞取残余饲料, 每天早晚定时用虹吸管清理粪便。高浓度氨氮暴露期间不投喂。实验期间水温(25.5±1.0)℃, 每天定时(18:30)更换预先配置好的相应质量浓度、等温的NH4Cl溶液的水, 用饱和Na2CO3溶液使水体pH维持稳定。
1.3 取样
在高浓度氨氮暴露前24h停止投喂。分别在高浓度氨氮暴露实验0、12h、48h和低浓度氨氮预适应3d、7d、14d后高浓度氨氮暴露实验12h和48h [对照组(C0; C12h; C48h)、预适应3d (PrE3d12h; PrE3d48h)、预适应7d (PrE7d12h; PrE7d48h)、预适应14d (PrE14d12h; PrE14d48h)], 每个时间点随机选取每池10条鱼(每组30条), 用120 mg/L MS-222。每个时间点收集鱼左侧全部鳃组织, 鳃组织样本处理: 将5条鱼的样本合并, 冷冻在液氮中, 用于后续的生理生化分析; 将4条鱼的样本合并, 冷冻在液氮中, 用于后续的RNA提取; 将1条鱼的样本固定在4%多聚甲醛中, 用于后续组织学分析。
1.4 组织学
将鳃组织取出, 用生理盐水清洗, 剪切后分装入管, 加入4%的多聚甲醛固定24h。在分级乙醇溶液中脱水, 在二甲苯中澄清, 并包埋在石蜡中。制备4 μm的切片, 用于苏木精和伊红(HE)染色, 用显微镜观察并分析。
1.5 免疫指标测定
用预冷生理盐水按1﹕9比例制成组织匀浆液, 在4℃, 9000 r/min条件下离心10min, 取鳃上清液。采用不同的合肥莱尔生物科技有限公司检测试剂盒测定鳃上清液中的免疫球蛋白M (ImmunoglobulinM, IgM)、补体C3 (Complement3, C3)、补体C4 (Complement4, C4)的含量和溶菌酶(Lysozyme, LZM)的酶活性。
1.6 基因表达水平测定
使用TRIzolplus™总RNA提取试剂在氨氮胁迫0、12h和48h时从黄颡鱼的鳃组织中提取总RNA。用1%琼脂糖凝胶电泳检测RNA的纯度和完整性。使用逆转录试剂盒逆转录成cDNA, 选择4个免疫因子进行定量实时聚合酶链反应(qPCR)分析。qPCR的引物序列如表 1所示, 扩增效率在90%以上。使用2×SYBR Green qPCR Mix (Antibody; Genenode, 武汉)在FS96实时PCR系统(常州福生生物技术有限公司, 江苏)中进行扩增。PCR扩增程序为: 95℃预变性3min, 95℃变性5s, 60℃退火-延伸30s, 40个循环。使用β-actin作为参考基因, 采用2–ΔΔCt法换算目的基因的相对表达量。
表 1 实时荧光定量PCR引物序列Table 1. Primer sequences for qPCR目标基因
Target gene引物序列
Primer sequence (5′—3′)登录号
Accession number补体3 (c3) ACCCATAGAAAAGAAGAGCGACT XM027136454 AAATCCTGTCAGCAAGCCGAT 免疫球蛋白M (igm) ACTGGCTCCACATACACCT JQ067604.1 CTTGCCATTTTCTCTTTTGTACTGA 白细胞介素1β (il-1β) TCAGCCTACAACCCACCAAA JQ730738 GCTCCATTCCATCGTTCTCCT 肿瘤坏死因子α (tnf-α) ATCTCAAGGGGACTTACTCCA XM_027146388.1 CCACCCCTTCAAGTAATTCACA 肌动蛋白(β-action) TGCTGCCTCTTCCTCCTCTC KM673246.1 GGACACCTGAACCTCTCATTGC 1.7 统计与分析
所得实验数据均以平均值±标准差表示, 使用SPSS27.0软件(IBM, NY, USA)对研究获得的数据进行单因素方差分析(One-way ANOVA), 随后采用Duncan进行多重比较, P<0.05表示差异显著。
2. 结果
2.1 低浓度氨氮预适应后高氨氮胁迫对黄颡鱼鳃组织结构的影响
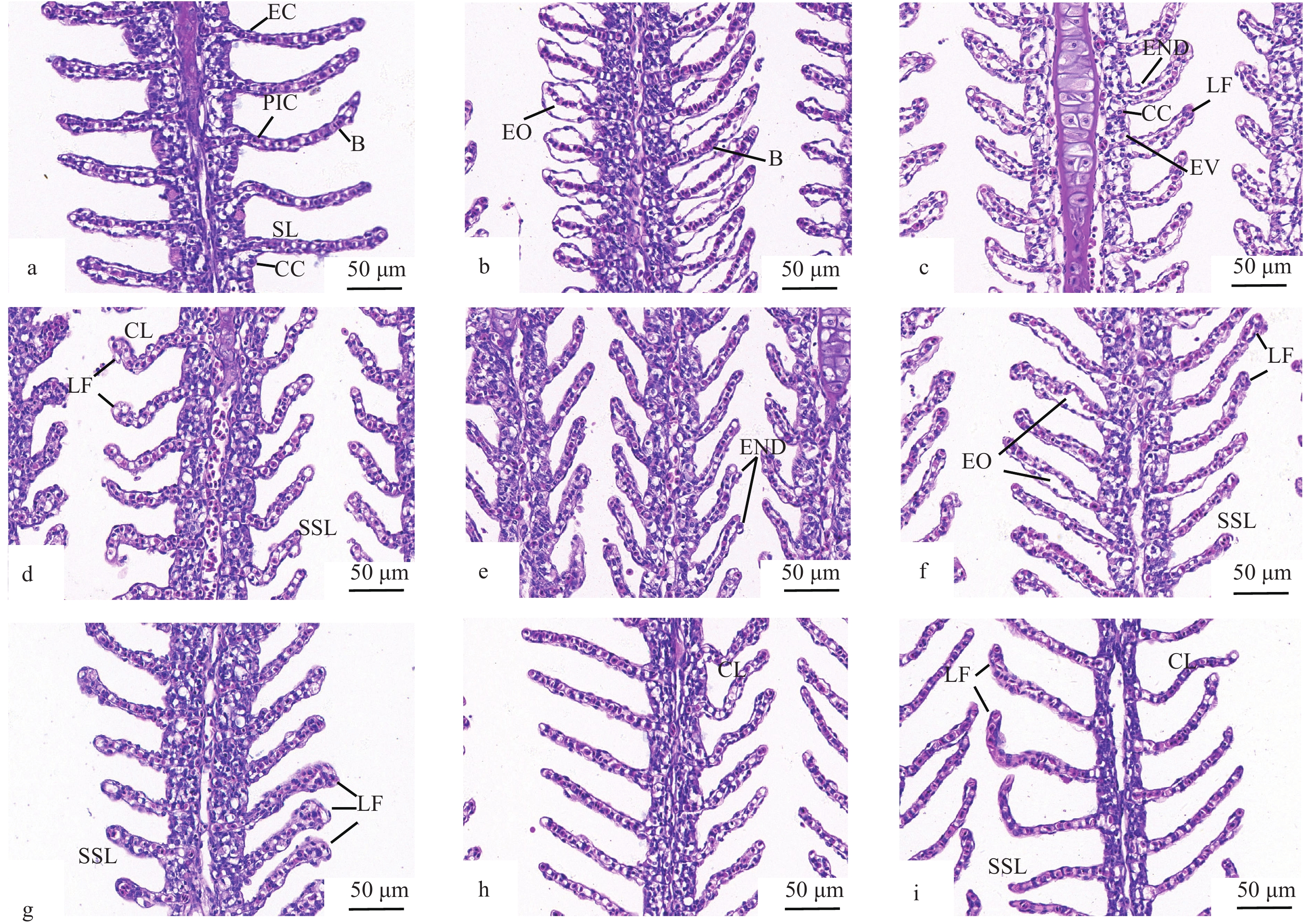
通过显微观察预适应组和对照组的鳃组织发现, 对照组(C0)鳃组织的鳃丝排列规则, 泌氯细胞和上皮细胞排列密集有序, 细胞核清晰, 鳃丝结构完整; 对照组(C12h)鳃组织出现上皮细胞水肿; 对照组(C48h)鳃组织的鳃丝之间的差距增大, 鳃丝基部充血萎缩, 上皮细胞排列紊乱, 上皮细胞和氯细胞空泡化, 鳃小片逐渐变短、卷曲、合并, 细胞空泡化严重(图 1)。在高浓度氨氮暴露12h时, 预适应3d (PrE3d12h)、7d (PrE7d12h)和14d (PrE14d12h)和对照组(C12h)鳃组织出现细胞空泡化, 鳃小片变短、卷曲、合并等不同程度的病理变化, 但预适应3d (PrE3d12h)、7d (PrE7d12h)和14d (PrE14d12h)鳃组织损伤程度均低于对照组(C12h); 相对于对照组(C12h)和预适应3d (PrE3d12h), 预适应7d (PrE7d12h)和14d (PrE14d12h)鳃组织损伤较轻, 其中14d (PrE14d12h)鳃组织损伤程度最小, 鳃丝排列较规则, 泌氯细胞和上皮细胞排列较密集有序, 细胞核清晰, 鳃小片出现轻微变短、卷曲、合并的现象。在高浓度氨氮暴露48h时, 预适应3d (PrE3d12h)、7d (PrE7d48h)和14d (PrE14d48h)和对照组(C48h)鳃组织的病理变化进一步加剧, 其中预适应14d (PrE14d48h)鳃组织损伤程度最小。
![]() 图 1 不同时长的低浓度氨氮预适应后高浓度氨氮下黄颡鱼鳃组织结构a. C0; b. C12h; c. C48h; d. PrE3d12h; e. PrE3d48h; f. PrE7d12h; g. PrE7d48h; h. PrE14d12h; i. PrE14d48h; EC. 上皮细胞; PIC. 柱状细胞; B. 血细胞; SL. 鳃小片; CC. 氯细胞; EO. 上皮细胞水肿; END. 上皮细胞坏死和脱落; LF. 层状融合; EV. 细胞空泡化; SSL. 鳃小片缩短; CL. 鳃小片卷曲Figure 1. Gill histological structure of yellow catfish under high ammonia concentration after pre- acclimatization to low ammonia concentration for different time periodsa. C0; b. C12h; c. C48h; d. PrE3d12h; e. PrE3d48h; f. PrE7d12h; g. PrE7d48h; h. PrE14d12h; i. PrE14d48h; EC. epithelial cells; PIC. pillar cells; B. blood cells; SL. secondary lamellae; CC. chloride cells; EO. epithelial oedema; END. epithelial necrosis and desquamation; LF. lamellae fusion; EV. cellular vacuolation; SSL. shortening of secondary lamellae; CL. curling of secondary lamellae
图 1 不同时长的低浓度氨氮预适应后高浓度氨氮下黄颡鱼鳃组织结构a. C0; b. C12h; c. C48h; d. PrE3d12h; e. PrE3d48h; f. PrE7d12h; g. PrE7d48h; h. PrE14d12h; i. PrE14d48h; EC. 上皮细胞; PIC. 柱状细胞; B. 血细胞; SL. 鳃小片; CC. 氯细胞; EO. 上皮细胞水肿; END. 上皮细胞坏死和脱落; LF. 层状融合; EV. 细胞空泡化; SSL. 鳃小片缩短; CL. 鳃小片卷曲Figure 1. Gill histological structure of yellow catfish under high ammonia concentration after pre- acclimatization to low ammonia concentration for different time periodsa. C0; b. C12h; c. C48h; d. PrE3d12h; e. PrE3d48h; f. PrE7d12h; g. PrE7d48h; h. PrE14d12h; i. PrE14d48h; EC. epithelial cells; PIC. pillar cells; B. blood cells; SL. secondary lamellae; CC. chloride cells; EO. epithelial oedema; END. epithelial necrosis and desquamation; LF. lamellae fusion; EV. cellular vacuolation; SSL. shortening of secondary lamellae; CL. curling of secondary lamellae2.2 低浓度氨氮预适应后高氨氮胁迫对黄颡鱼免疫指标的影响
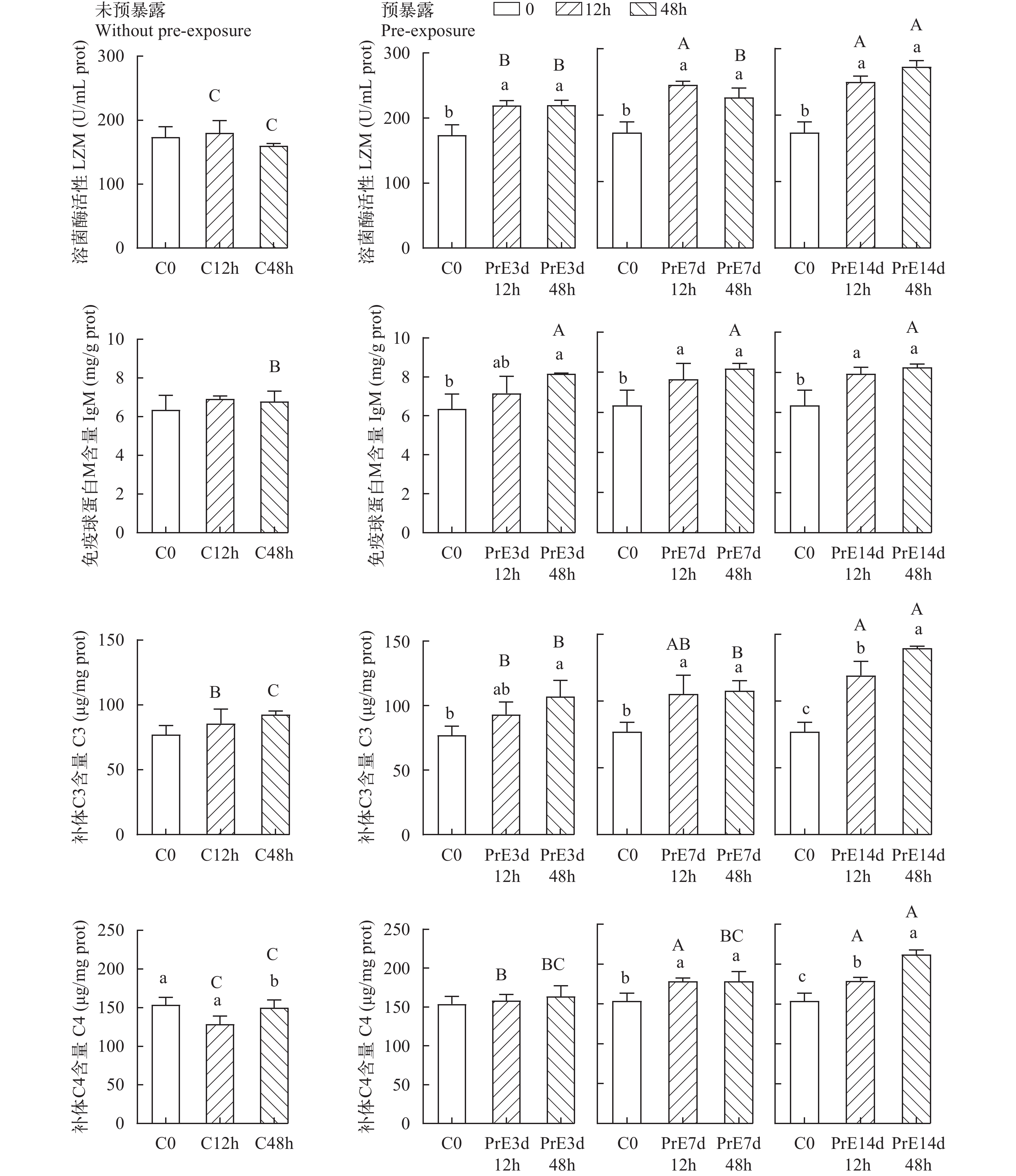
黄颡鱼预适应组和对照组鳃组织的4种免疫指标在高浓度氨氮暴露下变化如图 2所示, LZM 活性和IgM、C3含量在高浓度氨氮暴露48h后, 与对照组(C0)相比, 预适应3d (PrE3d12h; PrE3d48h)、7d (PrE7d12h; PrE7d48h)和14d (PrE14d12h; PrE14d48h)鳃组织内 LZM 活性和IgM、C3含量显著升高(P<0.05), 其中对照组(C12h; C48h)鳃组织内LZM 活性和IgM、C3含量没有显著差异(P>0.05), 预适应7d的 LZM 活性先升高后降低。C4含量在高浓度氨氮暴露48h后, 与对照组(C0)相比, 预适应7d (PrE7d12h; PrE7d48h)和14d (PrE14d12h; PrE14d48h)鳃组织内C4含量显著升高(P<0.05), 对照组(C48h)鳃组织内C4含量显著降低(P<0.05); 但3d (PrE3d12h; PrE3d48h)鳃组织内C4含量没有显著差异(P>0.05)。
![]() 图 2 低浓度氨氮预适应后高浓度氨氮下黄颡鱼的鳃组织免疫指标变化不同小写字母表示同一处理组内不同时间点显著性差异(P<0.05), 不同的大写字母表示高氨氮暴露同一时间点“未预暴露”与“预暴露”之间的显著性差异(P<0.05), 相同字母表示表明没有显著性差异(P>0.05)Figure 2. Changes in gill tissue immune indices of yellow catfish at high ammonia concentration after pre-acclimatization to low ammonia concentrationDifferent lowercase letters indicate significant differences (P<0.05) at different time points within the same treatment group, and different uppercase letters indicate significant differences (P<0.05) between “without pre-exposure” and “pre-exposure” at the same time point for high ammonia exposure, with the same letter indicating no significant difference (P>0.05). The same letter indicates that there is no significant difference (P>0.05)
图 2 低浓度氨氮预适应后高浓度氨氮下黄颡鱼的鳃组织免疫指标变化不同小写字母表示同一处理组内不同时间点显著性差异(P<0.05), 不同的大写字母表示高氨氮暴露同一时间点“未预暴露”与“预暴露”之间的显著性差异(P<0.05), 相同字母表示表明没有显著性差异(P>0.05)Figure 2. Changes in gill tissue immune indices of yellow catfish at high ammonia concentration after pre-acclimatization to low ammonia concentrationDifferent lowercase letters indicate significant differences (P<0.05) at different time points within the same treatment group, and different uppercase letters indicate significant differences (P<0.05) between “without pre-exposure” and “pre-exposure” at the same time point for high ammonia exposure, with the same letter indicating no significant difference (P>0.05). The same letter indicates that there is no significant difference (P>0.05)在高浓度氨氮暴露12h时, 预适应3d (PrE3d12h)、7d (PrE7d12h)和14d (PrE14d12h)的鳃组织内LZM活性和C3、 C4含量呈现显著升高趋势(P<0.05; 预适应3d (PrE3d12h)、7d (PrE7d12h)和14d (PrE14d12h)的LZM活性显著高于对照组(C12h; P<0.05), 其中预适应14d (PrE14d12h)最高; 预适应14d (PrE14d12h)的补体C3含量显著高于对照组(C12h)和3d (PrE3d12h; P<0.05); 预适应7d (PrE7d12h)和14d (PrE14d12h)的显著补体C4含量高于对照组(C12h)和预适应3d (PrE3d12h; P<0.05); 而IgM含量没有显著差异(P>0.05)。
在高浓度氨氮暴露48h时, 预适应3d (PrE3d48h)、7d (PrE7d48h)和14d (PrE14d48h)鳃组织内LZM活性和IgM、C3和C4含量显著升高(P<0.05); 预适应3d (PrE3d48h)、7d (PrE7d48h)和14d (PrE14d48h)的LZM活性显著高于对照组(C48h; P<0.05), 其中预适应14d (PrE14d48h)最高; 预适应3d (PrE3d48h)、7d (PrE7d48h)和14d (PrE14d48h)的IgM含量显著高于对照组(C48h; P<0.05); 预适应3d (PrE3d48h)、7d (PrE7d48h)和14d (PrE14d48h)的补体C3含量显著高于对照组(C48h; P<0.05), 其中预适应14d (PrE14d48h)最高; 预适应14d (PrE14d48h)的补体C4含量显著高于对照组(C48h)、预适应3d (PrE3d48h)和7d (PrE7d48h; P<0.05)。
2.3 低浓度氨氮预适应后高氨氮胁迫对炎症和免疫相关基因的影响
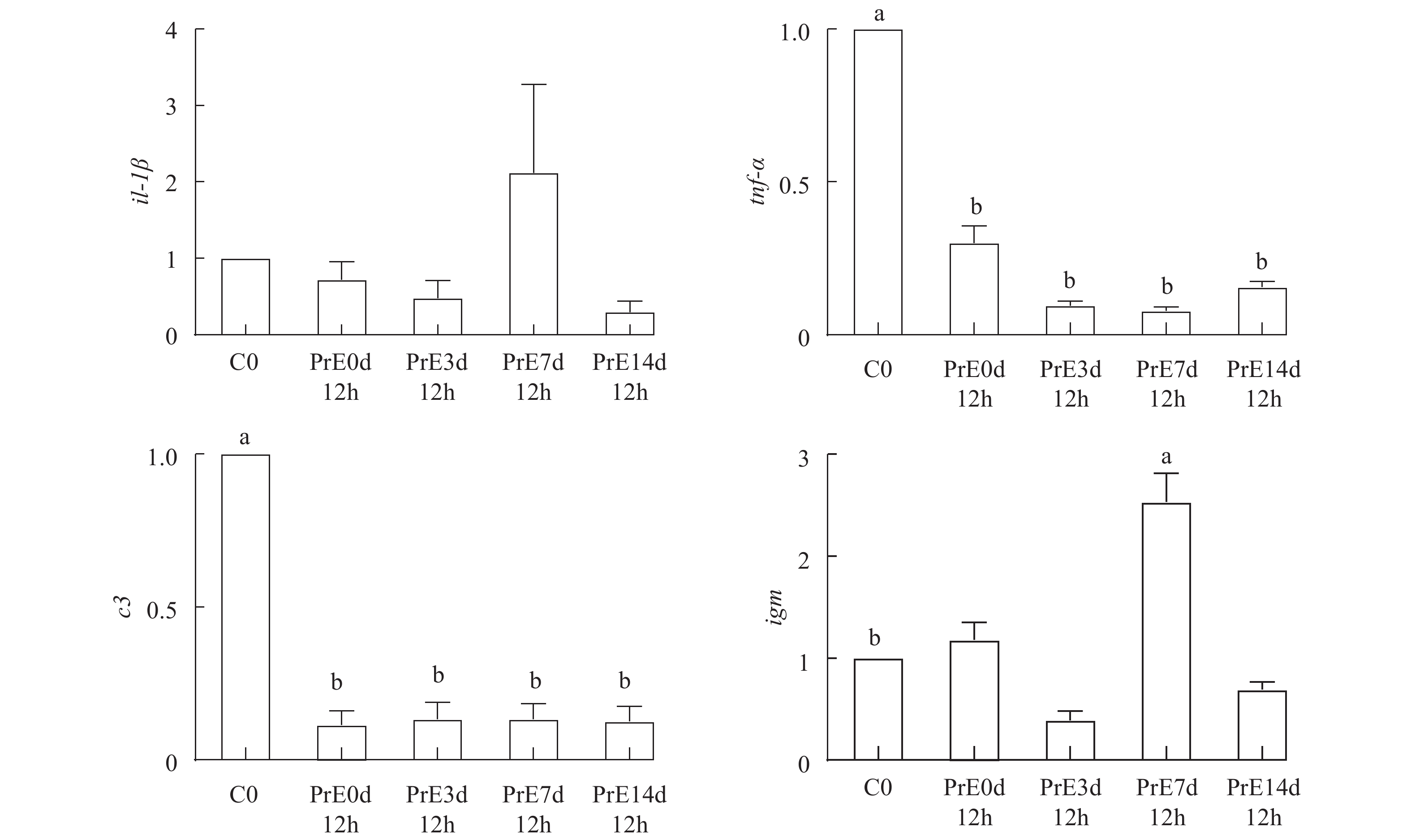
黄颡鱼预适应组和对照组鳃组织4个免疫相关基因在高浓度氨氮暴露下变化如图 3所示, 在高浓度氨氮暴露12h, 与对照组(C0)相比, 对照组(C12h)、预适应3d (PrE3d12h)、7d (PrE7d12h)和14d (PrE14d12h)鳃组织内il-1β基因表达水平没有显著差异(P>0.05), 而预适应7d(PrE7d12h)鳃组织内il-1β基因表达水平上调, 对照组(C12h)、预适应3d (PrE3d12h)和14d (PrE14d12h)鳃组织内il-1β基因表达水平下调。在高浓度氨氮暴露12h, 与对照组(C0)相比, 对照组(C12h)、预适应3d (PrE3d12h)、7d (PrE7d12h)和14 (PrE14d12h)鳃组织内tnf-α、c3基因表达水平呈现显著下调(P<0.05), 其中预适应7d (PrE7d12h)鳃组织内tnf-α基因表达水平最低。在高浓度氨氮暴露12h, 与对照组(C0)相比, 仅预适应7d (PrE7d12h)鳃组织内igm基因表达水平显著上调(P<0.05), 其中对照组(C12h)、预适应7d (PrE7d12h)鳃组织内igm基因表达水平上调, 预适应3d (PrE3d12h)和14d (PrE14d12h)鳃组织内igm基因表达水平下调。
![]() 图 3 低浓度氨氮预适应后高浓度氨氮下黄颡鱼鳃组织免疫相关基因的表达模式不同小写字母表示存在显著性差异(P<0.05)Figure 3. Expression patterns of immunity-related genes in gill tissues of yellow catfish under high ammonia concentration after pre- acclimatization to low ammonia concentrationDifferent lowercase letters indicate a significant difference (P<0.05)
图 3 低浓度氨氮预适应后高浓度氨氮下黄颡鱼鳃组织免疫相关基因的表达模式不同小写字母表示存在显著性差异(P<0.05)Figure 3. Expression patterns of immunity-related genes in gill tissues of yellow catfish under high ammonia concentration after pre- acclimatization to low ammonia concentrationDifferent lowercase letters indicate a significant difference (P<0.05)3. 讨论
3.1 低浓度氨氮预适应后高氨氮胁迫对黄颡鱼组织结构的影响
鳃是鱼类对不利环境条件做出快速反应的器官, 对水污染物敏感[19]; 同时, 鳃也是免疫器官, 鳃小片的卷曲和缩短有助于减少污染物的吸收, 起到防御作用[16]。上皮细胞水肿是对环境的防御反应和适应机制, 能降低鳃渗透性, 增加外界有害物质与机体血液间的距离[10]。本实验通过HE染色切片观察发现高浓度氨氮暴露对黄颡鱼鳃组织结构影响表现为上皮细胞水肿, 上皮细胞和氯细胞空泡化, 鳃小片逐渐变短、卷曲、合并, 细胞空泡化。研究报道, 氨氮胁迫常常促使鳃组织和细胞结构发生功能性变化, 在台湾泥鳅(Paramisgurnus dabryanus ssp. Taiwan)[10]、尼罗罗非鱼(Oreochromis nilotica)[20] 的氨氮胁迫实验中同样出现鳃组织上皮细胞水肿, 上皮细胞和氯细胞空泡化, 鳃小片逐渐变短、卷曲、合并, 细胞空泡化严重的现象。
预适应3d、7d和14d组鳃组织在高浓度氨氮暴露12h时的损伤程度均低于对照组(C12h), 说明经过不同时长的低浓度氨氮预适应后, 黄颡鱼的鳃组织对氨氮胁迫具有一定的适应性和抵抗能力, 其中预适应14d组鳃组织损伤程度最小, 鳃丝排列规则, 泌氯细胞和上皮细胞排列密集有序, 细胞核清晰, 仅鳃小片出现轻微的变短、卷曲、合并等现象。在高浓度氨氮暴露48h时, 预适应3d、7d和14d组与对照组(C48h)鳃组织均呈现明显损伤, 说明长时间在高浓度氨氮暴露仍会引起严重的组织损伤。其中预适应14d组在12h、48h时鳃组织损伤程度最小, 可能是14d的低浓度氨氮预适应很好的提高了鳃组织对高浓度氨氮的耐受性。
3.2 低浓度氨氮预适应后高氨氮胁迫对黄颡鱼免疫指标的影响
水体环境存在各种应激源, 会增加鱼类免疫系统功能障碍, 集约化养殖中氨氮对鱼类免疫的影响已成为热点问题[21, 22]。LZM是鱼类先天免疫系统的重要防御因子, 是可以反映鱼类免疫的常规指标, 负责裂解细菌细胞壁[23]。鱼类体内LZM的活性受到多种因素的影响, 包括应激源的类型、强度和持续时间[24]。硬骨鱼体液中的免疫球蛋白是适应性系统的主要组成部分, 作为鱼类免疫应答的重要标志物[25]。LZM和IgM通过识别和清除病原体, 同时激活机体的补体系统来维持鱼类的健康状态[25, 26]; C3和C4是补体系统的主要成分, 在抵抗病原感染和炎症反应方面起着重要作用[27]。在高浓度氨氮暴露48h后, 预适应3d、7d和14d组鳃组织内LZM活性都出现显著升高趋势, 表明经过低浓度氨氮预适应可以引发黄颡鱼对高浓度氨氮环境的非特异性免疫反应。预适应7d组的LZM活性在12h时呈现升高趋势, 这可能是高浓度氨氮暴露造成急性应激, 刺激免疫系统, 使机体的免疫功能急剧升高, 表现为免疫指标活性的升高, 而随后又在48h时呈现下降趋势, 说明长时间的氨氮胁迫会造成免疫抑制, 这与以前对团头鲂[28]的研究的结果相似, 氨氮胁迫造成LZM活性呈现先升高后下降的趋势。所有组的IgM含量均表现逐渐升高趋势, 其中预适应3d、7d和14d组IgM含量均显著升高, 表明随着环境氨氮浓度升高引起鱼体内的体液免疫反应。在本研究中, 高浓度氨氮刺激黄颡鱼鳃组织内LZM的合成和分泌, 增强LZM活性, 进而激活补体系统促进补体C3和C4活性上升, 加强非特异性免疫, 以应对氨氮带来的毒性作用; 所有组的C3含量与IgM的变化相似, 表明随着环境氨氮浓度升高引起鱼的非特异性免疫反应; 在高浓度氨氮环境下48h, 预适应7d和14d组的鱼体内C4含量均表现逐渐升高趋势, 可能是经过7d和14d的低浓度氨氮预适应在高浓度氨氮环境下可以快速引起鱼体内非特异性免疫反应。本研究结果与Shrivastava等[2]的结果一致, 预先暴露于低浓度氨的鲤在随后暴露于高浓度氨氮时, 耐氨能力显著改善。这些研究结果可能促进笔者考虑应激源通过预适应对鱼类的影响。例如, Zeng等[4]对大黄鱼的研究结果表明, 与未预适应的大黄鱼相比, 低浓度铜预适应的大黄鱼体内活性氧(ROS)更低, 炎症反应更强烈。Zheng等[29]对大黄鱼的研究结果显示, 低浓度锌预暴露可以减轻高锌诱导的氧化应激。曾霖等[30]对大黄鱼的研究显示, 低浓度盐预适应可以通过调节离子和物质运输、脂类代谢、细胞凋亡和非特异性免疫等来提高大黄鱼的低盐胁迫耐受性。
在高浓度氨氮暴露12h时, 预适应3d、7d和14d鳃组织内LZM 活性和C3、 C4含量呈现显著升高趋势, 表明高浓度氨氮胁迫会影响黄颡鱼先天性免疫功能; 而预适应14d组的LZM活性和C3、C4含量均显著高于其他组, 表明经过14d的低浓度氨氮预适应的黄颡鱼对高浓度氨氮环境的耐氨氮能力显著改善。在高浓度氨氮暴露48h时, 预适应3d、7d和14d鳃组织内LZM 活性和IgM、C3和C4含量显著升高, 表明高浓度氨氮胁迫48h时使机体的免疫功能升高, 高浓度氨氮胁迫影响黄颡鱼先天性和适应性免疫功能; 与高浓度氨氮暴露12h的结果相似, 预适应14d组的LZM活性和IgM、C3、C4含量均显著高于其他组, 表明经过14d的低浓度氨氮预适应的黄颡鱼对高氨氮环境的耐受能力显著改善。
3.3 低浓度氨氮预适应后高氨氮胁迫对黄颡鱼免疫相关基因表达的影响
肿瘤坏死因子是一种多能促炎细胞因子, 参与细胞凋亡和炎症反应, 并在应激反应中释放一系列促炎细胞因子, 包括白细胞介素和一氧化氮合酶[31]。本研究结果表明, 在高浓度氨氮暴露12h, 与对照组(C0)相比, 对照组(C12h)、预适应3d (PrE3d12h)、7d (PrE7d12h)和14d (PrE14d12h)鳃组织内il-1β基因表达水平均无显著变化, 但预适应7d (PrE7d12h)鳃组织内il-1β基因表达水平上调, 而对照组(C12h)、预适应3d (PrE3d12h)和14d (PrE14d12h)鳃组织内il-1β基因表达水平下调; 且对照组(C12h)、预适应3d (PrE3d12h)、7d (PrE7d12h)和14d (PrE14d12h)鳃组织内tnf-α基因表达水平均显著下调。在水体中氨氮浓度升高时, 鱼体内发生一系列应激反应而产生大量ROS, 造成氧化应激[32], 氨暴露通过诱导促炎细胞因子il-1β和tnf-α基因的转录激活炎症反应[33]。在本研究中, 氨氮胁迫使预适应7d (PrE7d12h)的il-1β基因表达与大弹涂鱼(Boleophthalmus pectinirostris)[34]相似, il-1β基因表达水平上调表明氨诱导细胞炎症反应, 分析il-1β基因表达水平上调应与毒性兴奋效应(Hormesis)有关, 可能是经过7d的低浓度氨氮预适应可以快速引起预适应7d (PrE7d12h)鳃组织内免疫反应; 而il-1β和tnf-α 基因表达水平的下调可能与在高浓度氨氮胁迫条件下机体受到较强的氧化应激, 免疫功能下降有关, 表现为通过下调这些基因来保护机体免受进一步的氧化损伤。c3属于先天免疫反应, 在介导免疫和炎症反应中发挥着关键作用[35]。本研究中c3基因表达水平与tnf-α基因变化相似, 均显著下调, 结果显示黄颡鱼的免疫力下降, 表明氨氮应激诱导了免疫毒性。免疫球蛋白(Ig)是鱼类特异性体液免疫应答中的重要组成部分, IgM主要负责识别、结合特异性抗原[36]; 对照组和预适应7d组鳃组织内igm基因表达水平上调表明急性氨氮应激会造成免疫球蛋白的升高, 而预适应3d和14d组鳃组织内igm基因表达水平下调表明免疫功能受到抑制。Li等[37]的研究报道, 急性氨氮刺激诱发瓦氏黄颡鱼(Pelteobagrus vachelli)的炎性反应, 主要表现为溶菌酶活性下降和总免疫球蛋白含量减少等免疫抑制现象。Gao等[38]对红鳍东方鲀(Takifugu rubripes)的研究结果显示, 急性氨氮暴露造成LZM、IgM、C3 和 C4 水平显著下降, 表明氨氮暴露可以刺激鱼类的免疫反应, 通过诱导免疫毒性在一定程度上抑制免疫功能。
4. 结论
本研究结合组织病理学、生理生化指标和分子生物学角度对黄颡鱼幼鱼阶段经过低浓度氨氮预适应后高浓度氨氮胁迫的氨氮毒性效应进行综合分析。高浓度氨氮导致黄颡鱼幼鱼鳃组织受到氧化损伤, 产生免疫炎症响应, 损害机体内酶的平衡, 影响机体免疫功能, 基因的转录水平和酶的合成。本研究结果显示, 黄颡鱼经过低浓度氨氮预适应对高浓度氨氮表现出一定的耐受性, 但经过长时间的高浓度氨氮暴露, 仍会导致黄颡鱼受到严重的氨氮应激损伤, 说明低浓度氨氮预适应后黄颡鱼有应对短时间内突发高浓度氨氮环境的能力。鉴于本研究, 养殖鱼类预适应于低浓度氨氮环境中14d, 有助于提高池塘养殖中鱼苗对突发高浓度氨氮环境的耐受性。
-
图 1 不同时长的低浓度氨氮预适应后高浓度氨氮下黄颡鱼鳃组织结构
a. C0; b. C12h; c. C48h; d. PrE3d12h; e. PrE3d48h; f. PrE7d12h; g. PrE7d48h; h. PrE14d12h; i. PrE14d48h; EC. 上皮细胞; PIC. 柱状细胞; B. 血细胞; SL. 鳃小片; CC. 氯细胞; EO. 上皮细胞水肿; END. 上皮细胞坏死和脱落; LF. 层状融合; EV. 细胞空泡化; SSL. 鳃小片缩短; CL. 鳃小片卷曲
Figure 1. Gill histological structure of yellow catfish under high ammonia concentration after pre- acclimatization to low ammonia concentration for different time periods
a. C0; b. C12h; c. C48h; d. PrE3d12h; e. PrE3d48h; f. PrE7d12h; g. PrE7d48h; h. PrE14d12h; i. PrE14d48h; EC. epithelial cells; PIC. pillar cells; B. blood cells; SL. secondary lamellae; CC. chloride cells; EO. epithelial oedema; END. epithelial necrosis and desquamation; LF. lamellae fusion; EV. cellular vacuolation; SSL. shortening of secondary lamellae; CL. curling of secondary lamellae
图 2 低浓度氨氮预适应后高浓度氨氮下黄颡鱼的鳃组织免疫指标变化
不同小写字母表示同一处理组内不同时间点显著性差异(P<0.05), 不同的大写字母表示高氨氮暴露同一时间点“未预暴露”与“预暴露”之间的显著性差异(P<0.05), 相同字母表示表明没有显著性差异(P>0.05)
Figure 2. Changes in gill tissue immune indices of yellow catfish at high ammonia concentration after pre-acclimatization to low ammonia concentration
Different lowercase letters indicate significant differences (P<0.05) at different time points within the same treatment group, and different uppercase letters indicate significant differences (P<0.05) between “without pre-exposure” and “pre-exposure” at the same time point for high ammonia exposure, with the same letter indicating no significant difference (P>0.05). The same letter indicates that there is no significant difference (P>0.05)
图 3 低浓度氨氮预适应后高浓度氨氮下黄颡鱼鳃组织免疫相关基因的表达模式
不同小写字母表示存在显著性差异(P<0.05)
Figure 3. Expression patterns of immunity-related genes in gill tissues of yellow catfish under high ammonia concentration after pre- acclimatization to low ammonia concentration
Different lowercase letters indicate a significant difference (P<0.05)
表 1 实时荧光定量PCR引物序列
Table 1 Primer sequences for qPCR
目标基因
Target gene引物序列
Primer sequence (5′—3′)登录号
Accession number补体3 (c3) ACCCATAGAAAAGAAGAGCGACT XM027136454 AAATCCTGTCAGCAAGCCGAT 免疫球蛋白M (igm) ACTGGCTCCACATACACCT JQ067604.1 CTTGCCATTTTCTCTTTTGTACTGA 白细胞介素1β (il-1β) TCAGCCTACAACCCACCAAA JQ730738 GCTCCATTCCATCGTTCTCCT 肿瘤坏死因子α (tnf-α) ATCTCAAGGGGACTTACTCCA XM_027146388.1 CCACCCCTTCAAGTAATTCACA 肌动蛋白(β-action) TGCTGCCTCTTCCTCCTCTC KM673246.1 GGACACCTGAACCTCTCATTGC -
[1] Linton T K, Morgan I J, Reid S D, et al. Long-term exposure to small temperature increase and sublethal ammonia in hardwater acclimated rainbow trout: does acclimation occur [J]? Aquatic Toxicology, 1998, 40 (2/3): 171-191.
[2] Shrivastava J, Sinha A K, Datta S N, et al. Pre-acclimation to low ammonia improves ammonia handling in common carp (Cyprinus carpio) when exposed subsequently to high environmental ammonia [J]. Aquatic Toxicology, 2016(180): 334-344. doi: 10.1016/j.aquatox.2016.10.017
[3] Khan F U, Younas W, Shang Y, et al. Effects of acclimation temperature on growth, physiology and thermal tolerance of the juvenile grass carp Ctenopharyngodon idella [J]. Aquaculture, 2024(581): 740421. doi: 10.1016/j.aquaculture.2023.740421
[4] Zeng L, Zhang J S, Zheng J L, et al. Pre-acclimation to low copper mitigated immunotoxic effects in spleen and head-kidney of large yellow croaker (Pseudosciaena crocea) when exposed subsequently to high copper [J]. Ecotoxicology and Environmental Safety, 2017(144): 54-61. doi: 10.1016/j.ecoenv.2017.06.018
[5] Adeyemi J A, Klerks P L. Occurrence of copper acclimation in the least killifish Heterandria formosa, and associated biochemical and physiological mechanisms [J]. Aquatic Toxicology, 2013(130/131): 51-57. doi: 10.1016/j.aquatox.2013.01.001
[6] Mangang Y A, Pandey P K. Hemato-biochemical responses and histopathological alterations in the gill and kidney tissues of Osteobrama belangeri (Valenciennes, 1844) exposed to different sub-lethal unionized ammonia [J]. Aquaculture, 2021(542): 736887. doi: 10.1016/j.aquaculture.2021.736887
[7] 周鑫, 董云伟, 王芳, 等. 急性氨氮胁迫对于草鱼sod和hsp90基因表达及鳃部结构的影响 [J]. 水生生物学报, 2013, 37(2): 321-328.] doi: 10.7541/2013.21 Zhou X, Dong Y W, Wang F, et al. The effect of high ammonia concentration on gill structure alternation and expression of sod and hsp90 genes in grass carp, Ctenopharyngodon idella [J]. Acta Hydrobiologica Sinica, 2013, 37(2): 321-328. [ doi: 10.7541/2013.21
[8] 李波, 樊启学, 杨凯, 等. 慢性氨氮胁迫对黄颡鱼摄食、生长及血液指标的影响 [J]. 应用与环境生物学报, 2011, 17(6): 824-828.] Li B, Fan Q X, Yang K, et al. Effects of chronic ammonia stress on foraging, growth, and haematological parameters of yellow catfish (Pelteobagrus fulvidraco) juveniles [J]. Chinese Journal of Applied & Environmental Biology, 2011, 17(6): 824-828. [
[9] 张云龙, 王光毅, 金慧, 等. 氨基酸代谢调控在大鳞副泥鳅应对氨暴露中的作用 [J]. 水生生物学报, 2019, 43(5): 1013-1020.] doi: 10.7541/2019.120 Zhang Y L, Wang G Y, Jin H, et al. The effects of ammonia exposure on amino acid metabolism in Chinese loach Paramisgurnus dabryanus [J]. Acta Hydrobiologica Sinica, 2019, 43(5): 1013-1020. [ doi: 10.7541/2019.120
[10] 王梦杰, 马本贺, 王玮欣, 等. 慢性氨氮胁迫对台湾泥鳅幼鱼生长、免疫及组织结构的影响 [J]. 水生生物学报, 2021, 45(2): 267-274.] doi: 10.7541/2021.2019.281 Wang M J, Ma B H, Wang W X, et al. Effects of chronic ammonia stress on growth, immunity and histological structure of juvenile Taiwan loach (Paramisgurnus dabryanus ssp. taiwan) [J]. Acta Hydrobiologica Sinica, 2021, 45(2): 267-274. [ doi: 10.7541/2021.2019.281
[11] 魏孟申, 郑涛, 路思琪, 等. 氨氮胁迫对大口黑鲈幼鱼组织结构、酶活及肠道微生物的影响 [J]. 水生生物学报, 2024, 48(1): 10-22.] doi: 10.7541/2023.2023.0054 Wei M S, Zheng T, Lu S Q, et al. Ammonia-n stress on tissue structure, enzyme activity and intestinal microbiota of Macropterus salmoides [J]. Acta Hydrobiologica Sinica, 2024, 48(1): 10-22. [ doi: 10.7541/2023.2023.0054
[12] Sinha A K, Matey V, Giblen T, et al. Gill remodeling in three freshwater teleosts in response to high environmental ammonia [J]. Aquatic Toxicology, 2014(155): 166-180. doi: 10.1016/j.aquatox.2014.06.018
[13] 管敏, 张德志, 唐大明. 慢性氨氮胁迫对史氏鲟幼鱼生长及其肝脏抗氧化、免疫指标的影响 [J]. 南方水产科学, 2020, 16(2): 36-42.] doi: 10.12131/20190191 Guan M, Zhang D Z, Tang D M. Effects of chronic ammonia stress on growth, antioxidative and immunity indices in liver of juvenile Acipenser schrenckii [J]. South China Fisheries Science, 2020, 16(2): 36-42. [ doi: 10.12131/20190191
[14] Yuning Z, Weixing C, Banghua X, et al. Ammonia toxicity in the bighead carp (Aristichthys nobilis): hematology, antioxidation, immunity, inflammation and stress [J]. Toxics, 2023, 11(3): 243-243. doi: 10.3390/toxics11030243
[15] 高金伟, 吴浩, 李绍明, 等. 氨氮和镉胁迫对芙蓉鲤鲫抗氧化系统和免疫机能的影响 [J]. 水生生物学报, 2022, 46(4): 448-456.] doi: 10.7541/2021.2020.282 Gao J W, Wu H, Li S M, et al. Ammonia nitrogen and cadmium stress on antioxidant system and immune function of Furong crucian carp (Cyprinus capio Furong. ♀ × Carassius auratus red var. ♂) [J]. Acta Hydrobiologica Sinica, 2022, 46(4): 448-456. [ doi: 10.7541/2021.2020.282
[16] Liu M J, Guo H Y, Liu B, et al. Gill oxidative damage caused by acute ammonia stress was reduced through the HIF-1α/NF-κb signaling pathway in golden pompano (Trachinotus ovatus) [J]. Ecotoxicology and Environmental Safety, 2021(222): 112504. doi: 10.1016/j.ecoenv.2021.112504
[17] 王晓南, 郑欣, 闫振广, 等. 水质基准鱼类受试生物筛选 [J]. 环境科学研究, 2014, 27(4): 349-348.] Wang X N, Zheng X, Yan Z G, et al. Screening of native fishes for deriving aquatic life criteria [J]. Research of Environmental Sciences, 2014, 27(4): 349-348. [
[18] 张磊. 黄颡鱼能量收支及生物能量学最适生长模型的研究 [D]. 武汉: 华中农业大学, 2010: 64.] Zhang L. Energy budget and optimum bioenergetic growth model of yellow catfish (Pelteobagrus fulvidraco) [D]. Wuhan: Huazhong agricultural university, 2010: 64. [
[19] Benli A C K, Köksal G, Ozkul A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): effects on gill, liver and kidney histology [J]. Chemosphere, 2008, 72(9): 1355-1358. doi: 10.1016/j.chemosphere.2008.04.037
[20] Esam F, Khalafalla M M, Gewaily M S, et al. Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia [J]. Ecotoxicology and Environmental Safety, 2022(231): 113187. doi: 10.1016/j.ecoenv.2022.113187
[21] 赵红霞, 乔国贤, 李培佳, 等. 饲料添加L-精氨酸或N-氨甲酰谷氨酸对黄颡鱼生长、肠道功能、血清生化指标和抗氨氮应激能力的影响 [J]. 动物营养学报, 2021, 33(11): 6330-6339.] doi: 10.3969/j.issn.1006-267x.2021.11.033 Zhang H X, Qiao G X, Li P J, et al. Effects of dietary L-arginine or N-carbamylglutamate on growth performance, intestianal functuon, serum biochemical indexes and anti-ammonia-nitrogen stress ability of yellow catfish (Pelteobagrus fulvidraco) [J]. Chinese Journal of Animal Nutrtion, 2021, 33(11): 6330-6339. [ doi: 10.3969/j.issn.1006-267x.2021.11.033
[22] 莫郁坚, 易成琳, 肖扬波, 等. 草鱼nrf1基因的克隆与表达及其应激响应研究 [J]. 水生生物学报, 2024, 48(3): 461-468.] doi: 10.7541/2024.2023.0231 Mo Y J, Yi C L, Xiao Y B, et al. Cloning, expression and stress response of nrf1 gene in grass carp (Ctenopharyngodon idella) [J]. Acta Hydrobiologica Sinica, 2024, 48(3): 461-468. [ doi: 10.7541/2024.2023.0231
[23] Ellis A E. Innate host defense mechanisms of fish against viruses and bacteria [J]. Developmental and Comparative Immunology, 2001, 25(8/9): 827-839.
[24] Fevolden S E, Røed K H, Fjalestad K. A combined salt and confinement stress enhances mortality in rainbow trout (Oncorhynchus mykiss) selected for high stress responsiveness [J]. Aquaculture, 2003, 216(1/2/3/4): 67-76.
[25] Magnadottir B. Immunological control of fish diseases [J]. Marine Biotechnology, 2010, 12(4): 361-379. doi: 10.1007/s10126-010-9279-x
[26] Saurabh S, Sahoo P. K. Lysozyme: an important defence molecule of fish innate immune system [J]. Aquaculture Research, 2008, 39(3): 223-239. doi: 10.1111/j.1365-2109.2007.01883.x
[27] Li X, Liu L, Zhang Y, et al. Toxic effects of chlorpyrifos on lysozyme activities, the contents of complement C3 and IgM, and IgM and complement C3 expressions in common carp (Cyprinus carpio L.) [J]. Chemosphere, 2013, 93(2): 428-433. doi: 10.1016/j.chemosphere.2013.05.023
[28] 张春暖. 果寡糖对两种鲂属鱼生长、免疫和抗应激的研究 [D]. 南京: 南京农业大学, 2015: 58-65.] Zhang C N. The Effects of fructooligosaccharide on growth, immune responses, antistress in two Megalobrama fishes [D]. Nanjing: Nanjing Agricultural University, 2015: 58-65. [
[29] Zheng J L, Zhu Q L, Wu C W, et al. Zinc acclimation mitigated high zinc induced oxidative stress by enhancing antioxidant defenses in large yellow croaker Pseudosciaena crocea [J]. Aquatic Toxicology, 2016(172): 21-29. doi: 10.1016/j.aquatox.2015.12.009
[30] 曾霖, 张惠, 宋炜, 等. 低盐驯化对低盐胁迫下大黄鱼转录组的影响 [J]. 水产学报, 2023, 47(6): 41-52.] Zeng L, Zhang H, Song W, et al. Effects of low-salt acclimation on the transcriptome of large yellow croaker (Larimichthys crocea) under hypo-saline stress [J]. Journal of Fisheries of China, 2023, 47(6): 41-52. [
[31] Sun Z, Wang S, Zhang M, et al. Chronic toxicity study of ammonia exposure in juvenile yellow catfish Pelteobagrus fulvidraco [J]. Aquaculture, 2023(567): 739266. doi: 10.1016/j.aquaculture.2023.739266
[32] Ching B, Chew S F, Wong W P, et al. Environmental ammonia exposure induces oxidative stress in gills and brain of Boleophthalmus boddarti (Mudskipper) [J]. Aquatic Toxicology, 2009, 95(3): 203-212. doi: 10.1016/j.aquatox.2009.09.004
[33] 吴雪阳, 郭红会, 况宇, 等. 氨氮胁迫下不同食性鱼类仔鱼抗氧化和非特异性免疫的差异响应 [J]. 水生生物学报, 2022, 46(8): 1237-1248.] doi: 10.7541/2022.2021.0180 Wu X Y, Guo H H, Kuang Y, et al. Antioxidant and non-specific immune responses of four species of fish larvae under ammonia stress [J]. Acta Hydrobiologica Sinica, 2022, 46(8): 1237-1248. [ doi: 10.7541/2022.2021.0180
[34] 宋美泽, 黎明, 李健, 等. 急性氨氮暴露对大弹涂鱼炎性反应相关基因表达的影响 [J]. 水产学报, 2018, 42(11): 1704-1710.] Song M Z, Li M, Li J, et al. Effects of acute ammonia exposure on gene involved in inflammation of mudskipper [J]. Journal of Fisheries of China, 2018, 42(11): 1704-1710. [
[35] Xue S, Chen S, Ge Y, et al. Regulation of glutathione on growth performance, biochemical parameters, non-specific immunity, and related genes of common carp (Cyprinus carpio) exposed to ammonia [J]. Aquaculture, 2022(546): 737241. doi: 10.1016/j.aquaculture.2021.737241
[36] 李肖霞, 齐志涛, 迟爽, 等. 硬骨鱼类鳃黏膜免疫相关分子的研究进展 [J]. 水产学报, 2023, 47(4): 3-15.] Li X X, Qi Z T, Chi S, et al. Progress in research of the immune-related molecules in gill mucosa of teleost fish [J]. Journal of Fisheries of China, 2023, 47(4): 3-15. [
[37] Li M, Yu N, Qin J G, et al. Effects of ammonia stress, dietary linseed oil and Edwardsiella ictaluri challenge on juvenile darkbarbel catfish Pelteobagrus vachelli [J]. Fish & Shellfish Immunology, 2014, 38(1): 158-165.
[38] Gao X, Wang X, Wang X, et al. Toxicity in Takifugu rubripes exposed to acute ammonia: Effects on immune responses, brain neurotransmitter levels, and thyroid endocrine hormones [J]. Ecotoxicology and Environmental Safety, 2022(244): 114050. doi: 10.1016/j.ecoenv.2022.114050
-
期刊类型引用(1)
1. 周向阳. 氨氮对黄颡鱼养殖的影响及解决办法. 乡村科技. 2024(17): 90-92 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载: