THE EXPRESSION ANALYSIS OF FBXO32 GENE IN LIVER OF TRAF6 MUTANT ZEBRAFISH
-
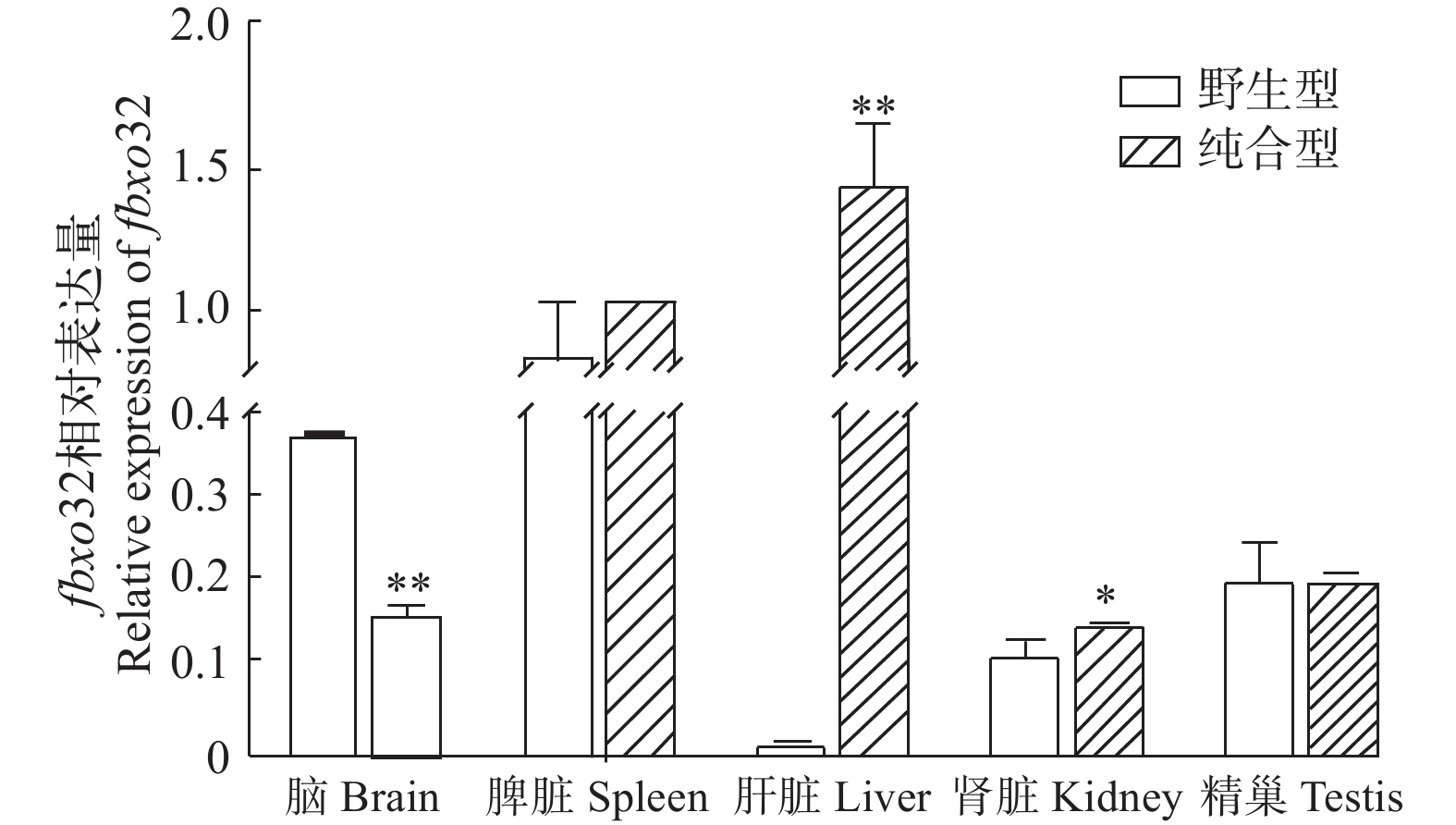
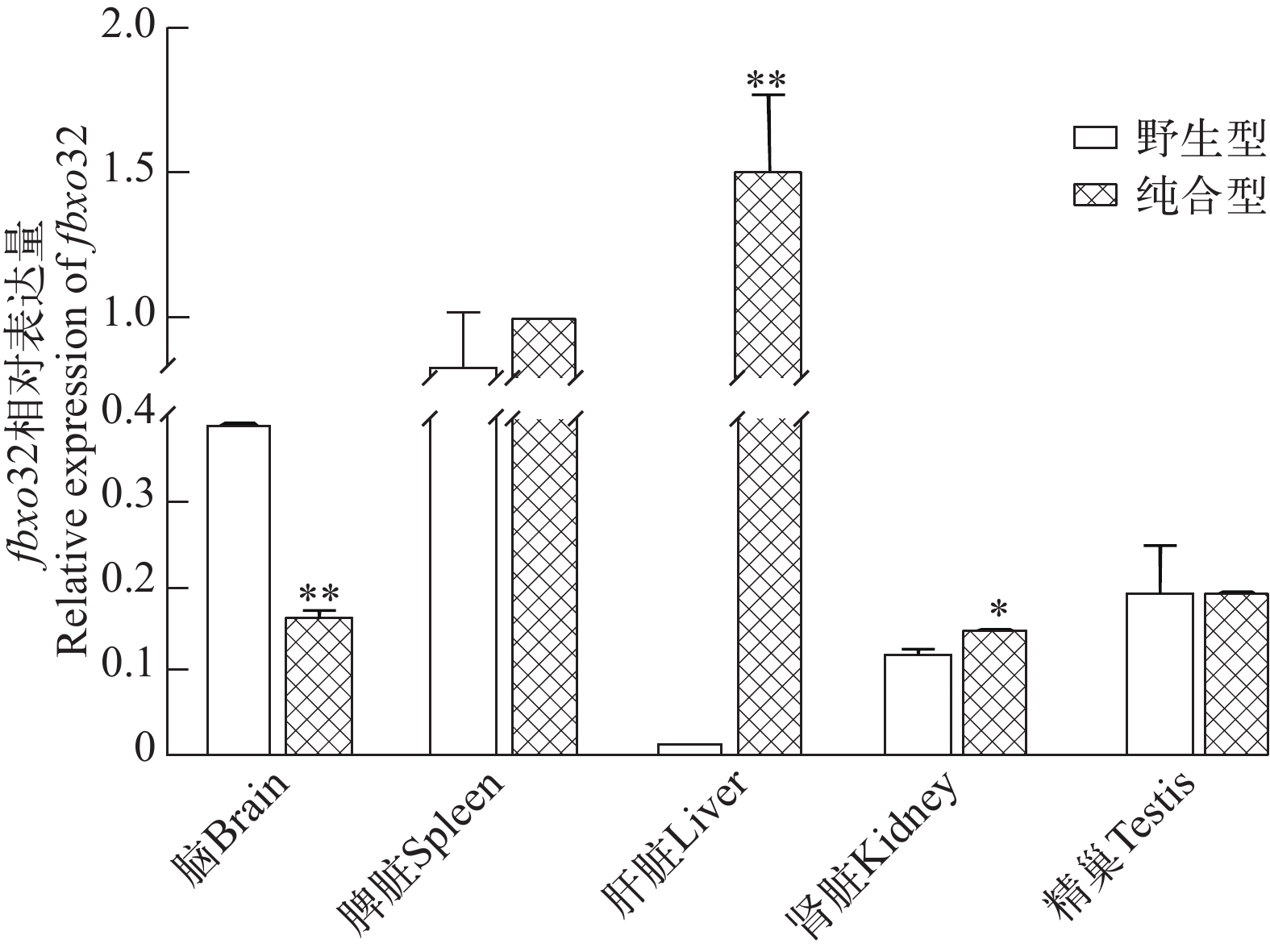
摘要: 为了进一步研究fbxo32在肝脏相关疾病中的作用, 进行了traf6缺失斑马鱼Danio rerio肝脏的组织切片分析, 结果显示斑马鱼traf6缺失个体表现出明显的肝萎缩特征, 包括肝脏组织结构松散、肝细胞排列不规则及缺少肝脂肪滴等症状。同时荧光定量实验表明fbxo32 mRNA在检测过的野生型斑马鱼组织中均有一定量的表达, 在肝脏中表达量较低而在卵巢中表达量较高。而与野生型斑马鱼相比, fbxo32 mRNA在traf6突变体斑马鱼肝脏中的表达量上调超过100倍。进一步原位杂交结果显示, fbxo32 mRNA的信号主要集中于肝脏细胞, 而在血细胞中则没有检测到信号。特别是与野生型斑马鱼相比, fbxo32 mRNA在traf6突变型斑马鱼肝脏中的信号明显增强。实验结果表明traf6缺失能引起fbxo32基因的上调表达, 并会导致traf6突变型斑马鱼肝脏发育异常并发生萎缩。Abstract: fbxo32, a muscle-specific E3 ubiquitin ligase, can enhance protein degradation to associate with atrophy. Here, we found that traf6 mutant zebrafish liver fbxo32 mRNA level increased dramatically. To find out the role of fbxo32 in liver disease, we performed histological analysis of mutant and wildtype zebrafish liver. The result showed that the mutant liver exhibited apparent characteristics of liver atrophy, such as loose liver tissue structure, irregular arrangement and rare lipid droplets of the hepatocytes. The qRT-PCR result showed that fbxo32 mRNA was widely expressed in most tested tissues with the highest level in ovary and low level in liver of wildtype zebrafish. Especially, compared with the wildtype, the liver fbxo32 mRNA was elevated about 100 folds in the traf6 mutant. Additionally, fbxo32 mRNA was mainly distributed in hepatocytes based on in situ hybridization, but cannot be detected in blood cells. The signal of fbxo32 mRNA was much stronger in traf6 mutant liver. These findings indicate that the knockout of traf6 might induce the expression of fbxo32 mRNA in liver and result in liver developmental abnormality and atrophy.
-
Keywords:
- Hepatic atrophy /
- fbxo32 /
- traf6 mutant /
- In situ hybridization /
- Zebrafish
-
-
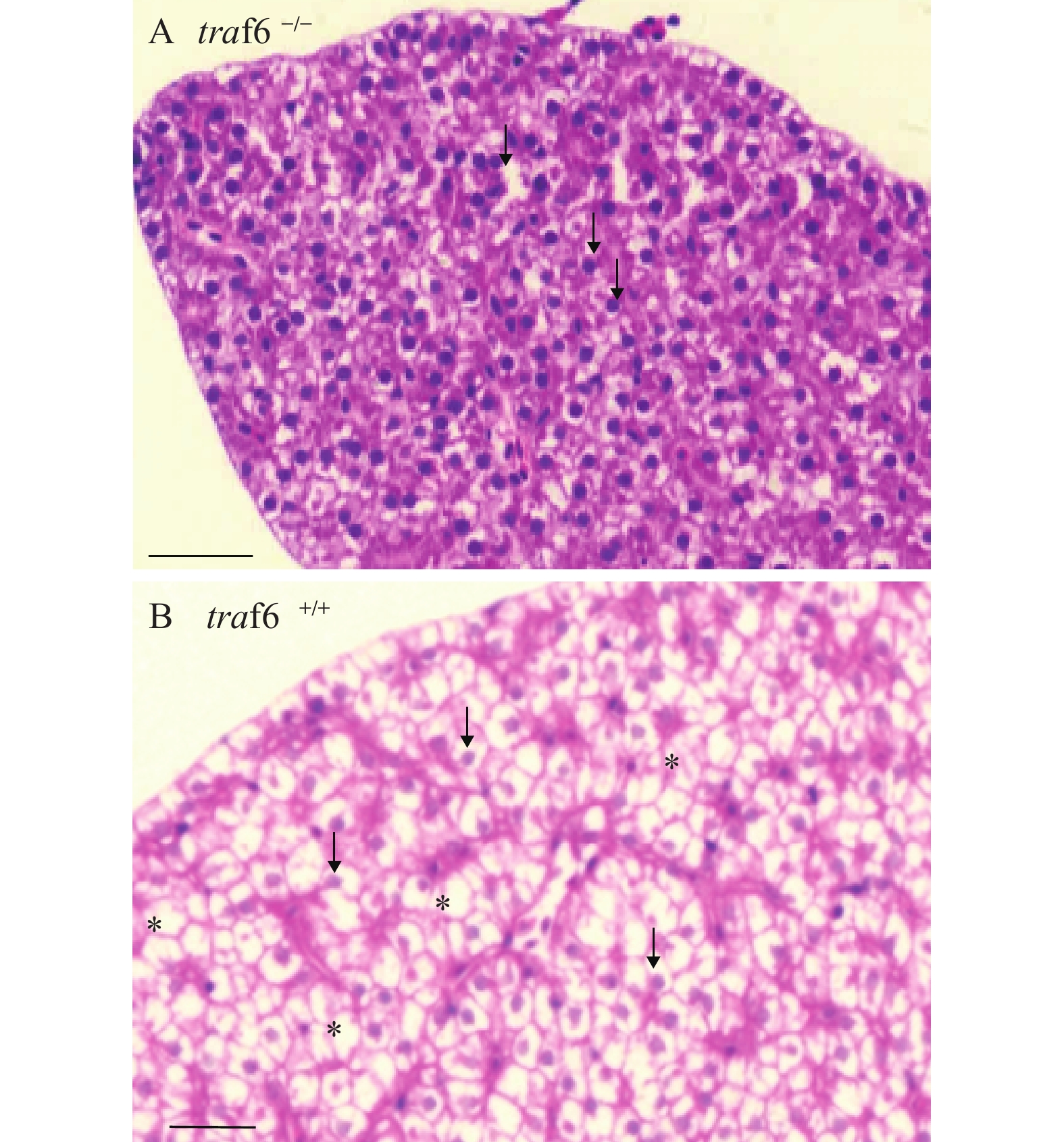
图 1 traf6缺失斑马鱼肝脏组织结构异常
2月龄斑马鱼肝脏组织横切面组织学分析, 通过石蜡包埋切片及苏木精伊红染色, 进行traf6缺失斑马鱼肝脏组织(A)与野生型肝脏组织(B)的比较分析。肝细胞核用箭头标示, 肝细胞脂肪滴用星号标示。比例尺, 20 μm
Figure 1. traf6 deficiency causes the abnormality of liver
The transverse sections of 2-month-old zebrafish liver. A, liver of traf6–/– zebrafish. B, liver of wild-type (wt) zebrafish. Liver nucleus were indicated by arrows. Stars for the Lipid droplets of hepatocytes. Scale bars, 20 μm
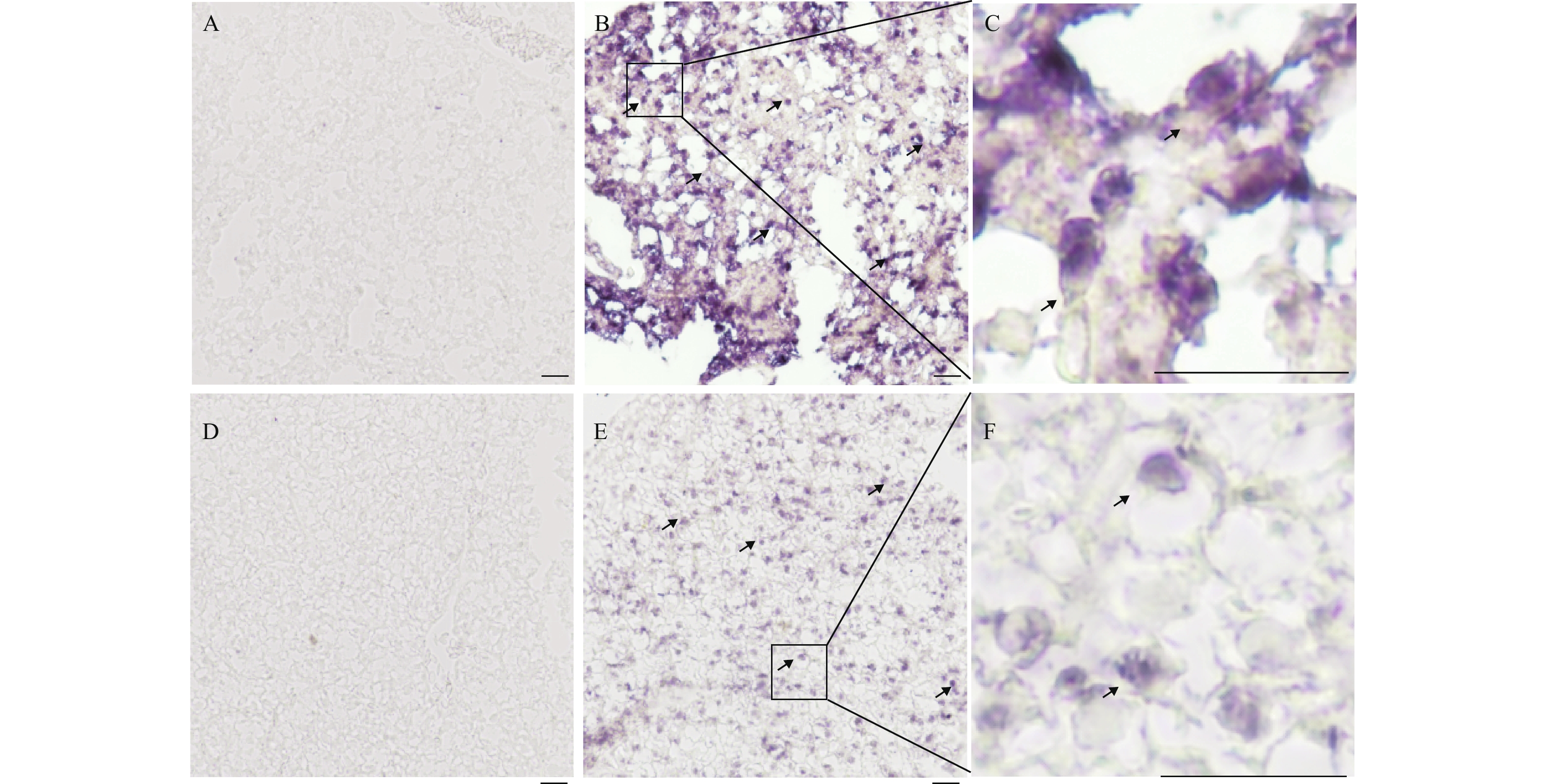
图 4 fbxo32转录本在肝脏组织中的表达水平
通过原位杂交技术用fbxo32正义探针(A, D)和反义探针(B, E)检测了fbxo32转录本在突变型斑马鱼(traf6–/–)和野生型斑马鱼(traf6+/+)中的表达。A、B. traf6–/–斑马鱼肝脏切片;D、E. traf6+/+斑马鱼肝脏切片;C、F. 阳性信号局部放大图。fbxo32的信号已用黑色箭头标出。比例尺, 20 μm
Figure 4. The expression level of fbxo32 mRNA in zebrafish liver tissue
The fbxo32 mRNA expression was examined by in situ hybridization on cryo-sections of livers in traf6 mutant (traf6–/–) and wildtype (traf6+/+) zebrafish with fbxo32 antisense (A, D) or sense probes (B, E). A, B. sections of traf6–/– zebrafish liver; D, E. sections of traf6+/+ zebrafish liver; C, F. drawing of partial enlargement. Signals of fbxo32 mRNA were highlighted with black arrows. Scale bars, 20 μm
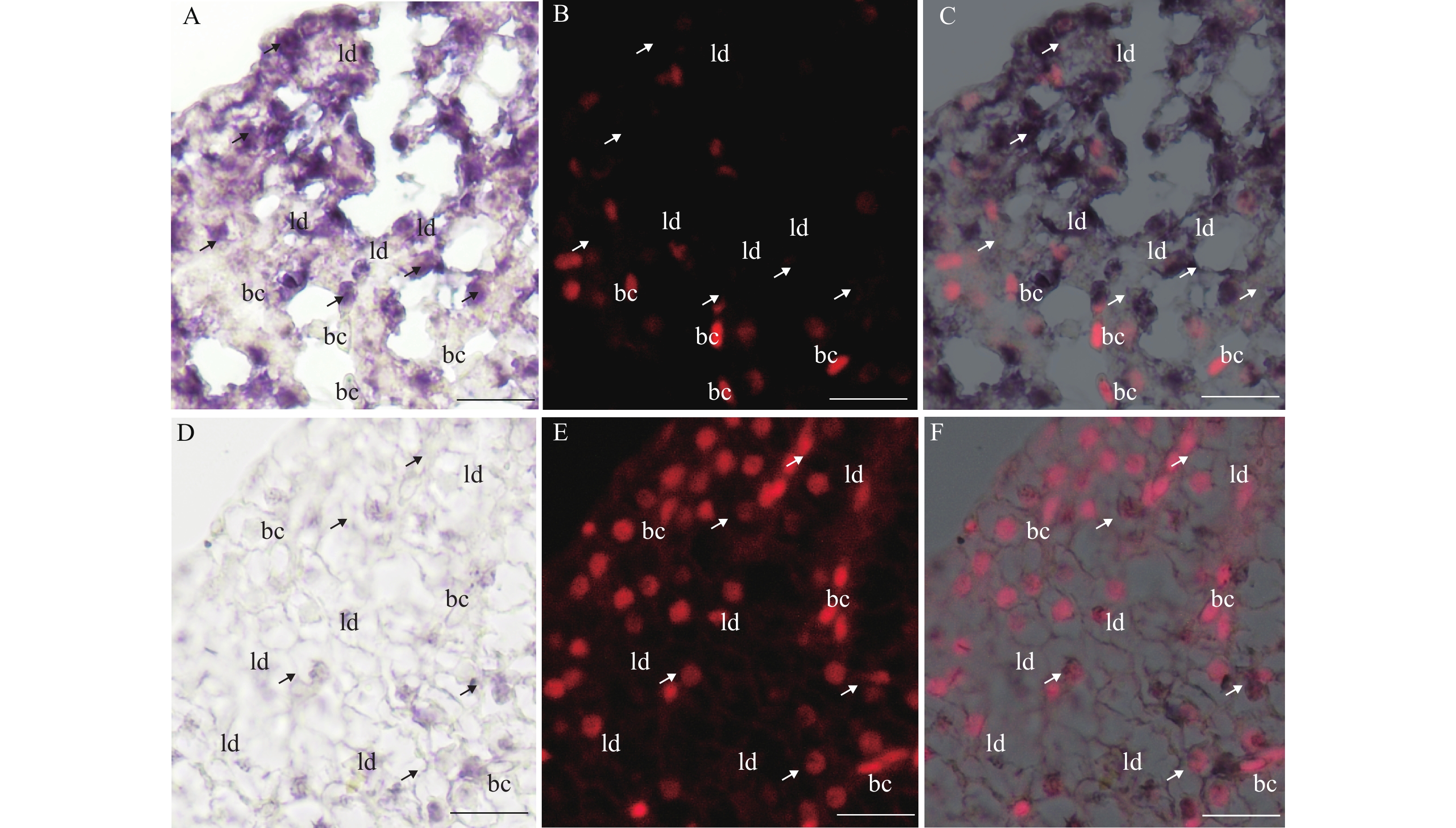
图 5 fbxo32转录本在斑马鱼肝脏组织中的细胞定位
2月龄斑马鱼肝脏组织的横向切片, 包括traf6–/–斑马鱼肝脏(A—C)和traf6+/+斑马鱼肝脏(D—F);紫色代表fbxo32转录本的信号;箭头指示的是肝脏细胞, 其细胞核较圆;bc. 血细胞, 细胞核长且梭型; ld. 肝脏细胞脂肪滴; 比例尺, 20 μm
Figure 5. The cellular distribution of fbxo32 mRNA in zebrafish liver tissues
The transverse sections of 2-month-old zebrafish livers, including the traf6–/– liver (A—C) and the traf6+/+ liver (D—F). The signals of fbxo32 mRNA were shown in purple. The arrows indicates the hepatocytes with round nuclear. bc, for blood cells with oral nuclear. ld, for the lipid droplets of hepatocytes. Scale bars, 20 μm
表 1 引物序列
Table 1 Primers list
引物名称
Primer name引物序列
Sequence (5′—3′)用途
Applicationfbxo32-F ACTGCCAATAACCCAGAGAGC 荧光定量 fbxo32-R CCCTGCCTCAAGTCATCCA 荧光定量 TZ-fbxo32-F ATTTAGGTGACACTATAGAAA
CTGCCAATAACCCAGAGAGC原位杂交 TZ-fbxo32-R TAATACGACTCACTATAGGG
CCCTGCCTCAAGTCATCCA原位杂交 -
[1] Ho M S, Tsai P I, Chien C T. F-box proteins: the key to protein degradation [J]. Journal of Biomedical Science, 2006, 13(2): 181-191. doi: 10.1007/s11373-005-9058-2
[2] Gomes M D, Lecker S H, Jagoe R T, et al. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy [J]. Proceedings of the National Academy of Sciences, 2001, 98(25): 14440-14445. doi: 10.1073/pnas.251541198
[3] Bodine S C, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy [J]. Science, 2001, 294(5547): 1704-1708. doi: 10.1126/science.1065874
[4] Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy [J]. Cell, 2004, 117(3): 399-412. doi: 10.1016/S0092-8674(04)00400-3
[5] Murdoch J D, Rostosky C M, Gowrisankaran S, et al. Endophilin-A deficiency induces the Foxo3a-Fbxo32 network in the brain and causes dysregulation of autophagy and the ubiquitin-proteasome system [J]. Cell Reports, 2016, 17(4): 1071-1086. doi: 10.1016/j.celrep.2016.09.058
[6] Nakashima K, Ishida A, Katsumata M. Comparison of proteolytic-related gene expression in the skeletal muscles of layer and broiler chickens [J]. Bioscience, Biotechnology and Biochemistry, 2009, 73(8): 1869-1871. doi: 10.1271/bbb.90134
[7] Cleveland B M, Evenhuis J P. Molecular characterization of atrogin-1/F-box protein-32 (FBXO32) and F-box protein-25 (FBXO25) in rainbow trout (Oncorhynchus mykiss): Expression across tissues in response to feed deprivation [J]. Comparative Biochemistry & Physiology Part B Biochemistry & Molecular Biology, 2010, 157(3): 248-257.
[8] Bühler A, Kustermann M, Bummer T, et al. Atrogin-1 deficiency leads to myopathy and heart failure in zebrafish [J]. International Journal of Molecular Sciences, 2016, 17(2): 187. doi: 10.3390/ijms17020187
[9] Howe K, Clark M D, Torroja C F, et al. The zebrafish reference genome sequence and its relationship to the human genome [J]. Nature, 2013, 496(7446): 498-503. doi: 10.1038/nature12111
[10] Lu J W, Ho Y J, Yang Y J, et al. Zebrafish as a disease model for studying human hepatocellular carcinoma [J]. World Journal of Gastroenterology, 2015, 21(42): 12042-12058. doi: 10.3748/wjg.v21.i42.12042
[11] Xu H, Li M, Gui J, et al. Cloning and expression of medaka dazl during embryogenesis and gametogenesis [J]. Gene Expression Patterns, 2007, 7(3): 332-338. doi: 10.1016/j.modgep.2006.08.001
[12] Tacchi L, Bickerdike R, Secombes C J, et al. Ubiquitin E3 ligase atrogin-1 (Fbox-32) in Atlantic salmon (Salmo salar): Sequence analysis, genomic structure and modulation of expression [J]. Comparative Biochemistry and Physiology. Part B,Biochemistry & Molecular Biology, 2010, 157(4): 364-373.
[13] Guo W, Zhang M, Shen S, et al. Aberrant methylation and decreased expression of the TGF-β/Smad target gene FBXO32 in esophageal squamous cell carcinoma [J]. Cancer, 2014, 120(16): 2412-2423. doi: 10.1002/cncr.28764
[14] Chou J L, Su H, Chen L, et al. Promoter hypermethylation of FBXO32, a novel TGF-β/SMAD4 target gene and tumor suppressor, is associated with poor prognosis in human ovarian cancer [J]. Laboratory Investigation, 2010, 90(3): 414-425. doi: 10.1038/labinvest.2009.138
[15] D′Azzo A, Bongiovanni A, Nastasi T. E3 Ubiquitin ligases as regulators of membrane protein trafficking and degradation [J]. Traffic, 2005, 6(6): 429-441. doi: 10.1111/j.1600-0854.2005.00294.x
[16] Sacheck J M, Hyatt J P K, Raffaello A, et al. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases [J]. The FASEB Journal, 2007, 21(1): 140-155. doi: 10.1096/fj.06-6604com
[17] Zhang P, Chen X, Fan M. Signaling mechanisms involved in disuse atrophy [J]. Medical Hypotheses, 2007, 69(2): 310-321. doi: 10.1016/j.mehy.2006.11.043
[18] 邝鸣, 刘晥蒙, 姚健, 等. 斑马鱼ftr56基因克隆表达及功能研究 [J]. 水生生物学报, 2020, 44(1): 20-25. doi: 10.7541/2020.003 Kuang M, Liu W M, Yao J, et al. Molecular cloning, eukaryotic expression and function study of ftr56 from Danio rerio [J]. Acta Hydrobiologica Sinica, 2020, 44(1): 20-25. doi: 10.7541/2020.003



 下载:
下载: